| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Endothelin receptor type B |
|---|
| Ligand | BDBM50061077 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_63852 (CHEMBL672282) |
|---|
| Ki | 111±n/a nM |
|---|
| Citation |  Liu, G; Henry, KJ; Szczepankiewicz, BG; Winn, M; Kozmina, NS; Boyd, SA; Wasicak, J; von Geldern, TW; Wu-Wong, JR; Chiou, WJ; Dixon, DB; Nguyen, B; Marsh, KC; Opgenorth, TJ Pyrrolidine-3-carboxylic acids as endothelin antagonists. 3. Discovery of a potent, 2-nonaryl, highly selective ETA antagonist (A-216546). J Med Chem41:3261-75 (1998) [PubMed] Article Liu, G; Henry, KJ; Szczepankiewicz, BG; Winn, M; Kozmina, NS; Boyd, SA; Wasicak, J; von Geldern, TW; Wu-Wong, JR; Chiou, WJ; Dixon, DB; Nguyen, B; Marsh, KC; Opgenorth, TJ Pyrrolidine-3-carboxylic acids as endothelin antagonists. 3. Discovery of a potent, 2-nonaryl, highly selective ETA antagonist (A-216546). J Med Chem41:3261-75 (1998) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Endothelin receptor type B |
|---|
| Name: | Endothelin receptor type B |
|---|
| Synonyms: | EDNRB | EDNRB_HUMAN | ENDOTHELIN B | ET-B | ETRB | Endothelin receptor ET-B | Endothelin receptor non-selective type | Endothelin receptor, ET-A/ET-B |
|---|
| Type: | Enzyme Catalytic Domain |
|---|
| Mol. Mass.: | 49664.00 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | ENDOTHELIN B EDNRB HUMAN::P24530 |
|---|
| Residue: | 442 |
|---|
| Sequence: | MQPPPSLCGRALVALVLACGLSRIWGEERGFPPDRATPLLQTAEIMTPPTKTLWPKGSNA
SLARSLAPAEVPKGDRTAGSPPRTISPPPCQGPIEIKETFKYINTVVSCLVFVLGIIGNS
TLLRIIYKNKCMRNGPNILIASLALGDLLHIVIDIPINVYKLLAEDWPFGAEMCKLVPFI
QKASVGITVLSLCALSIDRYRAVASWSRIKGIGVPKWTAVEIVLIWVVSVVLAVPEAIGF
DIITMDYKGSYLRICLLHPVQKTAFMQFYKTAKDWWLFSFYFCLPLAITAFFYTLMTCEM
LRKKSGMQIALNDHLKQRREVAKTVFCLVLVFALCWLPLHLSRILKLTLYNQNDPNRCEL
LSFLLVLDYIGINMASLNSCINPIALYLVSKRFKNCFKSCLCCWCQSFEEKQSLEEKQSC
LKFKANDHGYDNFRSSNKYSSS
|
|
|
|---|
| BDBM50061077 |
|---|
| n/a |
|---|
| Name | BDBM50061077 |
|---|
| Synonyms: | (1S,2R,3S)-1-Benzo[1,3]dioxol-5-yl-3-[2-(2-hydroxy-ethoxy)-4-methoxy-phenyl]-5-propoxy-indan-2-carboxylic acid | CHEMBL431651 | Enrasentan | SB 217242 | SB-217242 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C29H30O8 |
|---|
| Mol. Mass. | 506.5437 |
|---|
| SMILES | CCCOc1ccc2[C@@H]([C@H]([C@@H](c2c1)c1ccc(OC)cc1OCCO)C(O)=O)c1ccc2OCOc2c1 |
|---|
| Structure | 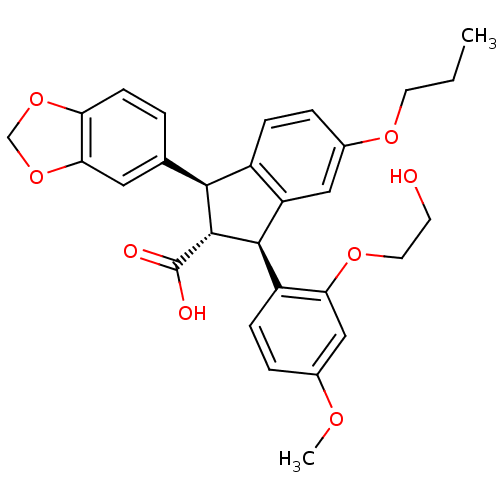
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Liu, G; Henry, KJ; Szczepankiewicz, BG; Winn, M; Kozmina, NS; Boyd, SA; Wasicak, J; von Geldern, TW; Wu-Wong, JR; Chiou, WJ; Dixon, DB; Nguyen, B; Marsh, KC; Opgenorth, TJ Pyrrolidine-3-carboxylic acids as endothelin antagonists. 3. Discovery of a potent, 2-nonaryl, highly selective ETA antagonist (A-216546). J Med Chem41:3261-75 (1998) [PubMed] Article
Liu, G; Henry, KJ; Szczepankiewicz, BG; Winn, M; Kozmina, NS; Boyd, SA; Wasicak, J; von Geldern, TW; Wu-Wong, JR; Chiou, WJ; Dixon, DB; Nguyen, B; Marsh, KC; Opgenorth, TJ Pyrrolidine-3-carboxylic acids as endothelin antagonists. 3. Discovery of a potent, 2-nonaryl, highly selective ETA antagonist (A-216546). J Med Chem41:3261-75 (1998) [PubMed] Article