| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Chymase |
|---|
| Ligand | BDBM50068889 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEBML_49442 |
|---|
| Ki | 6.3±n/a nM |
|---|
| Citation |  Eda, M; Ashimori, A; Akahoshi, F; Yoshimura, T; Inoue, Y; Fukaya, C; Nakajima, M; Fukuyama, H; Imada, T; Takai, S; Shiota, N; Miyazaki, M; Nakamura, N Peptidyl human heart chymase inhibitors. 1. Synthesis and inhibitory activity of difluoromethylene ketone derivatives bearing P' binding subsites. Bioorg Med Chem Lett8:913-8 (1999) [PubMed] Eda, M; Ashimori, A; Akahoshi, F; Yoshimura, T; Inoue, Y; Fukaya, C; Nakajima, M; Fukuyama, H; Imada, T; Takai, S; Shiota, N; Miyazaki, M; Nakamura, N Peptidyl human heart chymase inhibitors. 1. Synthesis and inhibitory activity of difluoromethylene ketone derivatives bearing P' binding subsites. Bioorg Med Chem Lett8:913-8 (1999) [PubMed] |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Chymase |
|---|
| Name: | Chymase |
|---|
| Synonyms: | Alpha-chymase | CMA1 | CMA1_HUMAN | CYH | CYM | Chymase precursor | Mast cell protease I |
|---|
| Type: | Enzyme |
|---|
| Mol. Mass.: | 27340.12 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | n/a |
|---|
| Residue: | 247 |
|---|
| Sequence: | MLLLPLPLLLFLLCSRAEAGEIIGGTECKPHSRPYMAYLEIVTSNGPSKFCGGFLIRRNF
VLTAAHCAGRSITVTLGAHNITEEEDTWQKLEVIKQFRHPKYNTSTLHHDIMLLKLKEKA
SLTLAVGTLPFPSQFNFVPPGRMCRVAGWGRTGVLKPGSDTLQEVKLRLMDPQACSHFRD
FDHNLQLCVGNPRKTKSAFKGDSGGPLLCAGVAQGIVSYGRSDAKPPAVFTRISHYRPWI
NQILQAN
|
|
|
|---|
| BDBM50068889 |
|---|
| n/a |
|---|
| Name | BDBM50068889 |
|---|
| Synonyms: | 3-(4-{[(S)-1-((S)-2-Benzenesulfonylamino-3-methyl-butyryl)-pyrrolidine-2-carbonyl]-amino}-2,2-difluoro-3-oxo-5-phenyl-pentanoylamino)-benzoic acid | CHEMBL170878 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C34H36F2N4O8S |
|---|
| Mol. Mass. | 698.733 |
|---|
| SMILES | CC(C)[C@H](NS(=O)(=O)c1ccccc1)C(=O)N1CCC[C@H]1C(=O)NC(Cc1ccccc1)C(=O)C(F)(F)C(=O)Nc1cccc(c1)C(O)=O |
|---|
| Structure | 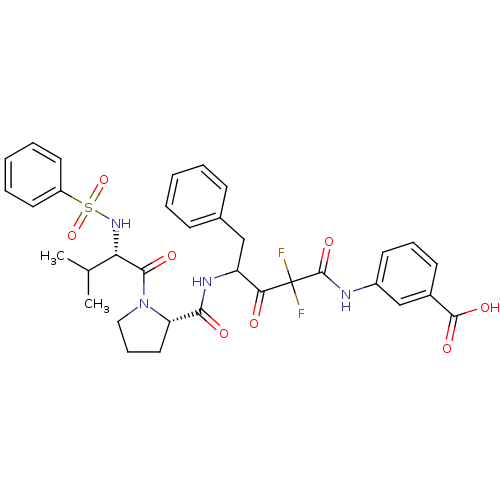
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Eda, M; Ashimori, A; Akahoshi, F; Yoshimura, T; Inoue, Y; Fukaya, C; Nakajima, M; Fukuyama, H; Imada, T; Takai, S; Shiota, N; Miyazaki, M; Nakamura, N Peptidyl human heart chymase inhibitors. 1. Synthesis and inhibitory activity of difluoromethylene ketone derivatives bearing P' binding subsites. Bioorg Med Chem Lett8:913-8 (1999) [PubMed]
Eda, M; Ashimori, A; Akahoshi, F; Yoshimura, T; Inoue, Y; Fukaya, C; Nakajima, M; Fukuyama, H; Imada, T; Takai, S; Shiota, N; Miyazaki, M; Nakamura, N Peptidyl human heart chymase inhibitors. 1. Synthesis and inhibitory activity of difluoromethylene ketone derivatives bearing P' binding subsites. Bioorg Med Chem Lett8:913-8 (1999) [PubMed]