| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Reverse transcriptase/RNaseH |
|---|
| Ligand | BDBM50071764 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_197451 (CHEMBL804236) |
|---|
| IC50 | 15000±n/a nM |
|---|
| Citation |  Milton, J; Slater, MJ; Bird, AJ; Spinks, D; Scott, G; Price, CE; Downing, S; Green, DV; Madar, S; Bethell, R; Stammers, DK Biaryl acids: novel non-nucleoside inhibitors of HIV reverse transcriptase types 1 and 2. Bioorg Med Chem Lett8:2623-8 (1999) [PubMed] Milton, J; Slater, MJ; Bird, AJ; Spinks, D; Scott, G; Price, CE; Downing, S; Green, DV; Madar, S; Bethell, R; Stammers, DK Biaryl acids: novel non-nucleoside inhibitors of HIV reverse transcriptase types 1 and 2. Bioorg Med Chem Lett8:2623-8 (1999) [PubMed] |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Reverse transcriptase/RNaseH |
|---|
| Name: | Reverse transcriptase/RNaseH |
|---|
| Synonyms: | HIV-1 Reverse Transcriptase RNase H | Human immunodeficiency virus type 1 reverse transcriptase | Reverse transcriptase/RNaseH |
|---|
| Type: | PROTEIN |
|---|
| Mol. Mass.: | 65229.15 |
|---|
| Organism: | Human immunodeficiency virus 1 |
|---|
| Description: | ChEMBL_1473730 |
|---|
| Residue: | 566 |
|---|
| Sequence: | PISPIETVPVKLKPGMDGPKVKQWPLTEEKIKALVEICTEMEKEGKISKIGPENPYNTPV
FAIKKKDSTKWRKLVDFRELNKRTQDFWEVQLGIPHPAGLKKRKSVTVLDVGDAYFSVPL
DEDFRKYTAFTIPSINNETPGIRYQYNVLPQGWKGSPAIFQSSMTKILEPFRKQNPDIVI
YQYMDDLYVGSDLEIGQHRTKIEELRQHLLRWGLTTPDKKHQKEPPFLWMGYELHPDKWT
VQPIVLPEKDSWTVNDIQKLVGKLNWASQIYPGIRVRQLCKLLRGTKALTEVIPLTEEAE
LELAENREILKEPVHGVYYDPSKDLIAEIQKQGQGQWTYQIYQEPFKNLRTGKYARMRGA
HTNDVKQLTEAVQKITTESIVIWGKTPKFKLPIQKETWETWWTEYWQATWIPEWEFVNTP
PLVKLWYQLEKEPIVGAETFYVDGAANRETKLGKAGYVTNRGRQKVVTLTDTTNQKTELQ
AIYLALQDSGLEVNIVTDSQYALGIIQAQPDQSESELVNQIIEQLIKKEKVYLAWVPAHK
GIGGNEQVDKLVSAGIRKVLFLDGID
|
|
|
|---|
| BDBM50071764 |
|---|
| n/a |
|---|
| Name | BDBM50071764 |
|---|
| Synonyms: | 5-Bromo-2-[5-(4-bromo-phenylcarbamoyl)-pentanoylamino]-benzoic acid | CHEMBL314121 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C19H18Br2N2O4 |
|---|
| Mol. Mass. | 498.165 |
|---|
| SMILES | OC(=O)c1cc(Br)ccc1NC(=O)CCCCC(=O)Nc1ccc(Br)cc1 |
|---|
| Structure | 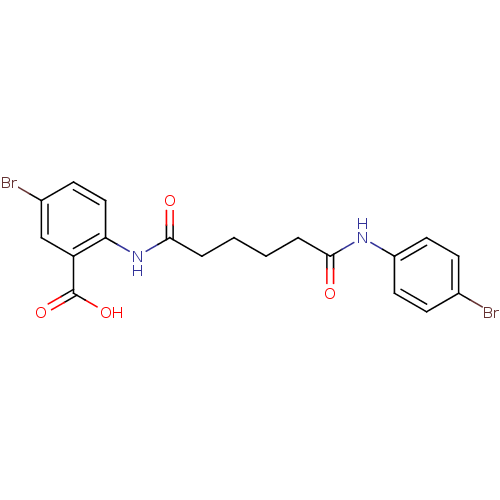
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Milton, J; Slater, MJ; Bird, AJ; Spinks, D; Scott, G; Price, CE; Downing, S; Green, DV; Madar, S; Bethell, R; Stammers, DK Biaryl acids: novel non-nucleoside inhibitors of HIV reverse transcriptase types 1 and 2. Bioorg Med Chem Lett8:2623-8 (1999) [PubMed]
Milton, J; Slater, MJ; Bird, AJ; Spinks, D; Scott, G; Price, CE; Downing, S; Green, DV; Madar, S; Bethell, R; Stammers, DK Biaryl acids: novel non-nucleoside inhibitors of HIV reverse transcriptase types 1 and 2. Bioorg Med Chem Lett8:2623-8 (1999) [PubMed]