| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Complement C1r subcomponent |
|---|
| Ligand | BDBM50063745 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_48026 (CHEMBL661599) |
|---|
| IC50 | 400±n/a nM |
|---|
| Citation |  Plummer, JS; Cai, C; Hays, SJ; Gilmore, JL; Emmerling, MR; Michael, W; Narasimhan, LS; Watson, MD; Wang, K; Nath, R; Evans, LM; Jaen, JC Benzenesulfonamide derivatives of 2-substituted 4H-3,1-benzoxazin-4-ones and benzthiazin-4-ones as inhibitors of complement C1r protease. Bioorg Med Chem Lett9:815-20 (1999) [PubMed] Plummer, JS; Cai, C; Hays, SJ; Gilmore, JL; Emmerling, MR; Michael, W; Narasimhan, LS; Watson, MD; Wang, K; Nath, R; Evans, LM; Jaen, JC Benzenesulfonamide derivatives of 2-substituted 4H-3,1-benzoxazin-4-ones and benzthiazin-4-ones as inhibitors of complement C1r protease. Bioorg Med Chem Lett9:815-20 (1999) [PubMed] |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Complement C1r subcomponent |
|---|
| Name: | Complement C1r subcomponent |
|---|
| Synonyms: | C1R | C1R_HUMAN | Complement C1r | Complement C1r subcomponent |
|---|
| Type: | Enzyme |
|---|
| Mol. Mass.: | 80113.65 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | P00736 |
|---|
| Residue: | 705 |
|---|
| Sequence: | MWLLYLLVPALFCRAGGSIPIPQKLFGEVTSPLFPKPYPNNFETTTVITVPTGYRVKLVF
QQFDLEPSEGCFYDYVKISADKKSLGRFCGQLGSPLGNPPGKKEFMSQGNKMLLTFHTDF
SNEENGTIMFYKGFLAYYQAVDLDECASRSKSGEEDPQPQCQHLCHNYVGGYFCSCRPGY
ELQEDTHSCQAECSSELYTEASGYISSLEYPRSYPPDLRCNYSIRVERGLTLHLKFLEPF
DIDDHQQVHCPYDQLQIYANGKNIGEFCGKQRPPDLDTSSNAVDLLFFTDESGDSRGWKL
RYTTEIIKCPQPKTLDEFTIIQNLQPQYQFRDYFIATCKQGYQLIEGNQVLHSFTAVCQD
DGTWHRAMPRCKIKDCGQPRNLPNGDFRYTTTMGVNTYKARIQYYCHEPYYKMQTRAGSR
ESEQGVYTCTAQGIWKNEQKGEKIPRCLPVCGKPVNPVEQRQRIIGGQKAKMGNFPWQVF
TNIHGRGGGALLGDRWILTAAHTLYPKEHEAQSNASLDVFLGHTNVEELMKLGNHPIRRV
SVHPDYRQDESYNFEGDIALLELENSVTLGPNLLPICLPDNDTFYDLGLMGYVSGFGVME
EKIAHDLRFVRLPVANPQACENWLRGKNRMDVFSQNMFCAGHPSLKQDACQGDSGGVFAV
RDPNTDRWVATGIVSWGIGCSRGYGFYTKVLNYVDWIKKEMEEED
|
|
|
|---|
| BDBM50063745 |
|---|
| n/a |
|---|
| Name | BDBM50063745 |
|---|
| Synonyms: | 2-(2-Iodo-phenylamino)-7-trifluoromethyl-benzo[d][1,3]oxazin-4-one | CHEMBL10312 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C15H8F3IN2O2 |
|---|
| Mol. Mass. | 432.1359 |
|---|
| SMILES | FC(F)(F)c1ccc2c(c1)nc(Nc1ccccc1I)oc2=O |
|---|
| Structure | 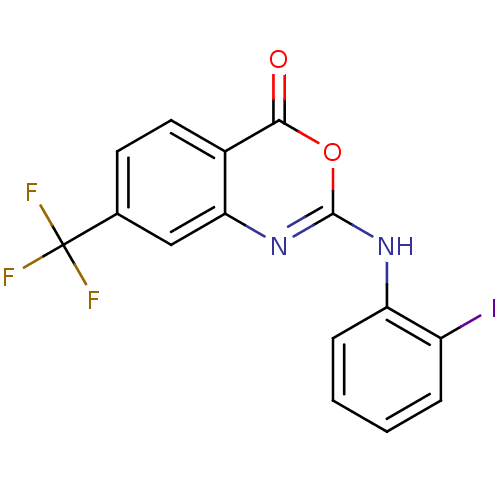
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Plummer, JS; Cai, C; Hays, SJ; Gilmore, JL; Emmerling, MR; Michael, W; Narasimhan, LS; Watson, MD; Wang, K; Nath, R; Evans, LM; Jaen, JC Benzenesulfonamide derivatives of 2-substituted 4H-3,1-benzoxazin-4-ones and benzthiazin-4-ones as inhibitors of complement C1r protease. Bioorg Med Chem Lett9:815-20 (1999) [PubMed]
Plummer, JS; Cai, C; Hays, SJ; Gilmore, JL; Emmerling, MR; Michael, W; Narasimhan, LS; Watson, MD; Wang, K; Nath, R; Evans, LM; Jaen, JC Benzenesulfonamide derivatives of 2-substituted 4H-3,1-benzoxazin-4-ones and benzthiazin-4-ones as inhibitors of complement C1r protease. Bioorg Med Chem Lett9:815-20 (1999) [PubMed]