| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Purine nucleoside phosphorylase |
|---|
| Ligand | BDBM50081806 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_162011 (CHEMBL764862) |
|---|
| IC50 | 37±n/a nM |
|---|
| Citation |  Yokomatsu, T; Hayakawa, Y; Suemune, K; Kihara, T; Soeda, S; Shimeno, H; Shibuya, S Synthesis and biological evaluation of 1,1-difluoro-2-(tetrahydro-3-furanyl)ethylphosphonic acids possessing a N9-purinylmethyl functional group at the ring. a new class of inhibitors for purine nucleoside phosphorylases. Bioorg Med Chem Lett9:2833-6 (1999) [PubMed] Yokomatsu, T; Hayakawa, Y; Suemune, K; Kihara, T; Soeda, S; Shimeno, H; Shibuya, S Synthesis and biological evaluation of 1,1-difluoro-2-(tetrahydro-3-furanyl)ethylphosphonic acids possessing a N9-purinylmethyl functional group at the ring. a new class of inhibitors for purine nucleoside phosphorylases. Bioorg Med Chem Lett9:2833-6 (1999) [PubMed] |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Purine nucleoside phosphorylase |
|---|
| Name: | Purine nucleoside phosphorylase |
|---|
| Synonyms: | Inosine phosphorylase | Inosine-guanosine phosphorylase | NP | PNP | PNPH_HUMAN | Purine nucleoside phosphorylase (PNPase) |
|---|
| Type: | Enzyme |
|---|
| Mol. Mass.: | 32119.53 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | n/a |
|---|
| Residue: | 289 |
|---|
| Sequence: | MENGYTYEDYKNTAEWLLSHTKHRPQVAIICGSGLGGLTDKLTQAQIFDYGEIPNFPRST
VPGHAGRLVFGFLNGRACVMMQGRFHMYEGYPLWKVTFPVRVFHLLGVDTLVVTNAAGGL
NPKFEVGDIMLIRDHINLPGFSGQNPLRGPNDERFGDRFPAMSDAYDRTMRQRALSTWKQ
MGEQRELQEGTYVMVAGPSFETVAECRVLQKLGADAVGMSTVPEVIVARHCGLRVFGFSL
ITNKVIMDYESLEKANHEEVLAAGKQAAQKLEQFVSILMASIPLPDKAS
|
|
|
|---|
| BDBM50081806 |
|---|
| n/a |
|---|
| Name | BDBM50081806 |
|---|
| Synonyms: | CHEMBL94920 | {1,1-Difluoro-2-[(3R,4S)-4-(6-oxo-1,6-dihydro-purin-9-ylmethyl)-tetrahydro-furan-3-yl]-ethyl}-phosphonic acid |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C12H15F2N4O5P |
|---|
| Mol. Mass. | 364.2419 |
|---|
| SMILES | OP(O)(=O)C(F)(F)C[C@H]1COC[C@@H]1Cn1cnc2c1nc[nH]c2=O |
|---|
| Structure | 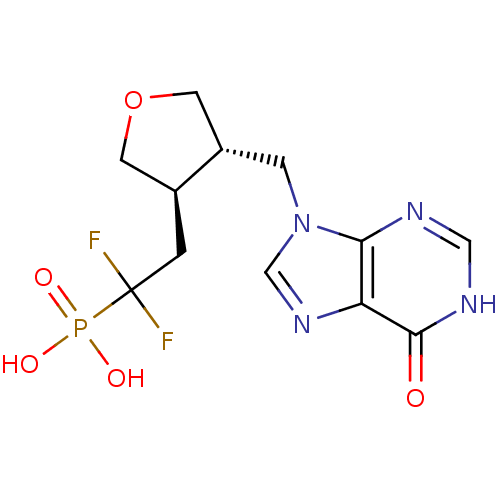
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Yokomatsu, T; Hayakawa, Y; Suemune, K; Kihara, T; Soeda, S; Shimeno, H; Shibuya, S Synthesis and biological evaluation of 1,1-difluoro-2-(tetrahydro-3-furanyl)ethylphosphonic acids possessing a N9-purinylmethyl functional group at the ring. a new class of inhibitors for purine nucleoside phosphorylases. Bioorg Med Chem Lett9:2833-6 (1999) [PubMed]
Yokomatsu, T; Hayakawa, Y; Suemune, K; Kihara, T; Soeda, S; Shimeno, H; Shibuya, S Synthesis and biological evaluation of 1,1-difluoro-2-(tetrahydro-3-furanyl)ethylphosphonic acids possessing a N9-purinylmethyl functional group at the ring. a new class of inhibitors for purine nucleoside phosphorylases. Bioorg Med Chem Lett9:2833-6 (1999) [PubMed]