| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Adenosine receptor A2a |
|---|
| Ligand | BDBM50086166 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_30015 (CHEMBL641306) |
|---|
| Ki | 25.9±n/a nM |
|---|
| Citation |  Kim, YC; Ji, X; Melman, N; Linden, J; Jacobson, KA Anilide derivatives of an 8-phenylxanthine carboxylic congener are highly potent and selective antagonists at human A(2B) adenosine receptors. J Med Chem43:1165-72 (2000) [PubMed] Kim, YC; Ji, X; Melman, N; Linden, J; Jacobson, KA Anilide derivatives of an 8-phenylxanthine carboxylic congener are highly potent and selective antagonists at human A(2B) adenosine receptors. J Med Chem43:1165-72 (2000) [PubMed] |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Adenosine receptor A2a |
|---|
| Name: | Adenosine receptor A2a |
|---|
| Synonyms: | AA2AR_RAT | ADENOSINE A2a | Adenosine A2 receptor | Adenosine A2a receptor (A2a) | Adenosine Receptors A2a (A2a) | Adenosine receptor A2a and A3 | Adenosine receptors A2a | Adora2a | Rat striatal adenosine A2a receptor |
|---|
| Type: | G Protein-Coupled Receptor (GPCR) |
|---|
| Mol. Mass.: | 45015.65 |
|---|
| Organism: | Rattus norvegicus (rat) |
|---|
| Description: | Rat A2A receptors expressed in CHO cells. |
|---|
| Residue: | 410 |
|---|
| Sequence: | MGSSVYITVELAIAVLAILGNVLVCWAVWINSNLQNVTNFFVVSLAAADIAVGVLAIPFA
ITISTGFCAACHGCLFFACFVLVLTQSSIFSLLAIAIDRYIAIRIPLRYNGLVTGVRAKG
IIAICWVLSFAIGLTPMLGWNNCSQKDGNSTKTCGEGRVTCLFEDVVPMNYMVYYNFFAF
VLLPLLLMLAIYLRIFLAARRQLKQMESQPLPGERTRSTLQKEVHAAKSLAIIVGLFALC
WLPLHIINCFTFFCSTCRHAPPWLMYLAIILSHSNSVVNPFIYAYRIREFRQTFRKIIRT
HVLRRQEPFQAGGSSAWALAAHSTEGEQVSLRLNGHPLGVWANGSATHSGRRPNGYTLGL
GGGGSAQGSPRDVELPTQERQEGQEHPGLRGHLVQARVGASSWSSEFAPS
|
|
|
|---|
| BDBM50086166 |
|---|
| n/a |
|---|
| Name | BDBM50086166 |
|---|
| Synonyms: | CHEMBL275605 | N-Benzyl-2-[4-(2,6-dioxo-1,3-dipropyl-2,3,6,7-tetrahydro-1H-purin-8-yl)-phenoxy]-acetamide | N-benzyl-2-(4-(2,6-dioxo-1,3-dipropyl-2,3,6,7-tetrahydro-1H-purin-8-yl)phenoxy)acetamide |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C26H29N5O4 |
|---|
| Mol. Mass. | 475.5396 |
|---|
| SMILES | CCCn1c2nc([nH]c2c(=O)n(CCC)c1=O)-c1ccc(OCC(=O)NCc2ccccc2)cc1 |
|---|
| Structure | 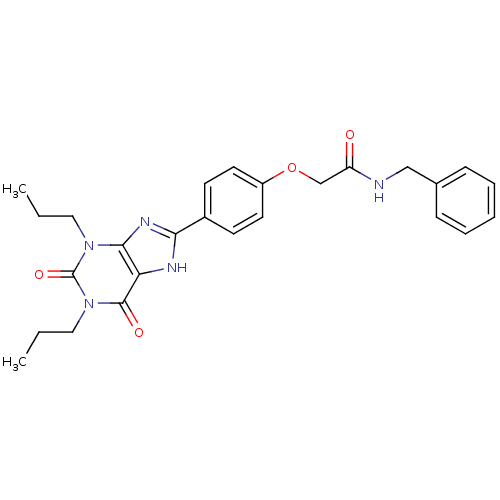
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Kim, YC; Ji, X; Melman, N; Linden, J; Jacobson, KA Anilide derivatives of an 8-phenylxanthine carboxylic congener are highly potent and selective antagonists at human A(2B) adenosine receptors. J Med Chem43:1165-72 (2000) [PubMed]
Kim, YC; Ji, X; Melman, N; Linden, J; Jacobson, KA Anilide derivatives of an 8-phenylxanthine carboxylic congener are highly potent and selective antagonists at human A(2B) adenosine receptors. J Med Chem43:1165-72 (2000) [PubMed]