| Reaction Details |
|---|
 | Report a problem with these data |
| Target | C-C chemokine receptor type 5 |
|---|
| Ligand | BDBM50088307 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_39630 (CHEMBL649937) |
|---|
| IC50 | 840.0±n/a nM |
|---|
| Citation |  Shiraishi, M; Aramaki, Y; Seto, M; Imoto, H; Nishikawa, Y; Kanzaki, N; Okamoto, M; Sawada, H; Nishimura, O; Baba, M; Fujino, M Discovery of novel, potent, and selective small-molecule CCR5 antagonists as anti-HIV-1 agents: synthesis and biological evaluation of anilide derivatives with a quaternary ammonium moiety. J Med Chem43:2049-63 (2000) [PubMed] Shiraishi, M; Aramaki, Y; Seto, M; Imoto, H; Nishikawa, Y; Kanzaki, N; Okamoto, M; Sawada, H; Nishimura, O; Baba, M; Fujino, M Discovery of novel, potent, and selective small-molecule CCR5 antagonists as anti-HIV-1 agents: synthesis and biological evaluation of anilide derivatives with a quaternary ammonium moiety. J Med Chem43:2049-63 (2000) [PubMed] |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| C-C chemokine receptor type 5 |
|---|
| Name: | C-C chemokine receptor type 5 |
|---|
| Synonyms: | C-C CKR-5 | C-C chemokine receptor type 5 | CC-CKR-5 | CCR-5 | CCR5 | CCR5/mu opioid receptor complex | CCR5_HUMAN | CD_antigen=CD195 | CHEMR13 | CMKBR5 | HIV-1 fusion coreceptor |
|---|
| Type: | Enzyme |
|---|
| Mol. Mass.: | 40540.21 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | P51681 |
|---|
| Residue: | 352 |
|---|
| Sequence: | MDYQVSSPIYDINYYTSEPCQKINVKQIAARLLPPLYSLVFIFGFVGNMLVILILINCKR
LKSMTDIYLLNLAISDLFFLLTVPFWAHYAAAQWDFGNTMCQLLTGLYFIGFFSGIFFII
LLTIDRYLAVVHAVFALKARTVTFGVVTSVITWVVAVFASLPGIIFTRSQKEGLHYTCSS
HFPYSQYQFWKNFQTLKIVILGLVLPLLVMVICYSGILKTLLRCRNEKKRHRAVRLIFTI
MIVYFLFWAPYNIVLLLNTFQEFFGLNNCSSSNRLDQAMQVTETLGMTHCCINPIIYAFV
GEKFRNYLLVFFQKHIAKRFCKCCSIFQQEAPERASSVYTRSTGEQEISVGL
|
|
|
|---|
| BDBM50088307 |
|---|
| n/a |
|---|
| Name | BDBM50088307 |
|---|
| Synonyms: | CHEMBL292345 | Triethyl-{4-[(7-phenyl-3,4-dihydro-naphthalene-2-carbonyl)-amino]-benzyl}-ammonium; chloride |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C30H35N2O |
|---|
| Mol. Mass. | 439.6112 |
|---|
| SMILES | CC[N+](CC)(CC)Cc1ccc(NC(=O)C2=Cc3cc(ccc3CC2)-c2ccccc2)cc1 |t:15| |
|---|
| Structure | 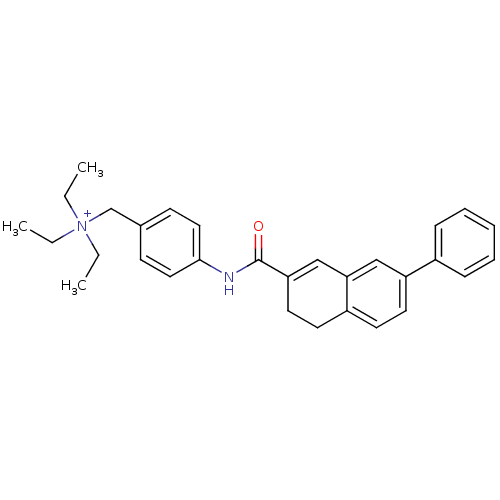
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Shiraishi, M; Aramaki, Y; Seto, M; Imoto, H; Nishikawa, Y; Kanzaki, N; Okamoto, M; Sawada, H; Nishimura, O; Baba, M; Fujino, M Discovery of novel, potent, and selective small-molecule CCR5 antagonists as anti-HIV-1 agents: synthesis and biological evaluation of anilide derivatives with a quaternary ammonium moiety. J Med Chem43:2049-63 (2000) [PubMed]
Shiraishi, M; Aramaki, Y; Seto, M; Imoto, H; Nishikawa, Y; Kanzaki, N; Okamoto, M; Sawada, H; Nishimura, O; Baba, M; Fujino, M Discovery of novel, potent, and selective small-molecule CCR5 antagonists as anti-HIV-1 agents: synthesis and biological evaluation of anilide derivatives with a quaternary ammonium moiety. J Med Chem43:2049-63 (2000) [PubMed]