| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Bifunctional dihydrofolate reductase-thymidylate synthase |
|---|
| Ligand | BDBM50090062 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_216586 (CHEMBL821378) |
|---|
| Ki | 1.4±n/a nM |
|---|
| Citation |  Yuthavong, Y; Vilaivan, T; Chareonsethakul, N; Kamchonwongpaisan, S; Sirawaraporn, W; Quarrell, R; Lowe, G Development of a lead inhibitor for the A16V+S108T mutant of dihydrofolate reductase from the cycloguanil-resistant strain (T9/94) of Plasmodium falciparum. J Med Chem43:2738-44 (2000) [PubMed] Yuthavong, Y; Vilaivan, T; Chareonsethakul, N; Kamchonwongpaisan, S; Sirawaraporn, W; Quarrell, R; Lowe, G Development of a lead inhibitor for the A16V+S108T mutant of dihydrofolate reductase from the cycloguanil-resistant strain (T9/94) of Plasmodium falciparum. J Med Chem43:2738-44 (2000) [PubMed] |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Bifunctional dihydrofolate reductase-thymidylate synthase |
|---|
| Name: | Bifunctional dihydrofolate reductase-thymidylate synthase |
|---|
| Synonyms: | Dihydrofolate Reductase-Thymidylate Synthase (DHFR-TS) Mutant KICB1 |
|---|
| Type: | n/a |
|---|
| Mol. Mass.: | 71741.43 |
|---|
| Organism: | Plasmodium falciparum |
|---|
| Description: | n/a |
|---|
| Residue: | 608 |
|---|
| Sequence: | MMEQVCDVFDIYAICACCKVESKNEGKKNEVFNNYTFRGLGNKGVLPWKCNSLDMKYFCA
VTTYVNESKYEKLKYKRCKYLNKETVDNVNDMPNSKKLQNVVVMGRTSWESIPKKFKPLS
NRINVILSRTLKKEDFDEDVYIINKVEDLIVLLGKLNYYKCFIIGGSVVYQEFLEKKLIK
KIYFTRINSTYECDVFFPEINENEYQIISVSDVYTSNNTTLDFIIYKKTNNKMLNEQNCI
KGEEKNNDMPLKNDDKDTCHMKKLTEFYKNVDKYKINYENDDDDEEEDDFVYFNFNKEKE
EKNKNSIHPNDFQIYNSLKYKYHPEYQYLNIIYDIMMNGNKQSDRTGVGVLSKFGYIMKF
DLSQYFPLLTTKKLFLRGIIEELLWFIRGETNGNTLLNKNVRIWEANGTREFLDNRKLFH
REVNDLGPIYGFQWRHFGAEYTNMYDNYENKGVDQLKNIINLIKNDPTSRRILLCAWNVK
DLDQMALPPCHILCQFYVFDGKLSCIMYQRSCDLGLGVPFNIASYSIFTHMIAQVCNLQP
AQFIHVLGNAHVYNNHIDSLKIQLNRIPYPFPTLKLNPDIKNIEDFTISDFTIQNYVHHE
KISMDMAA
|
|
|
|---|
| BDBM50090062 |
|---|
| n/a |
|---|
| Name | BDBM50090062 |
|---|
| Synonyms: | 1-(3,4-Dichloro-phenyl)-6-methyl-1,6-dihydro-[1,3,5]triazine-2,4-diamine | CHEMBL93153 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C10H11Cl2N5 |
|---|
| Mol. Mass. | 272.134 |
|---|
| SMILES | CC1N=C(N)N=C(N)N1c1ccc(Cl)c(Cl)c1 |t:2,5| |
|---|
| Structure | 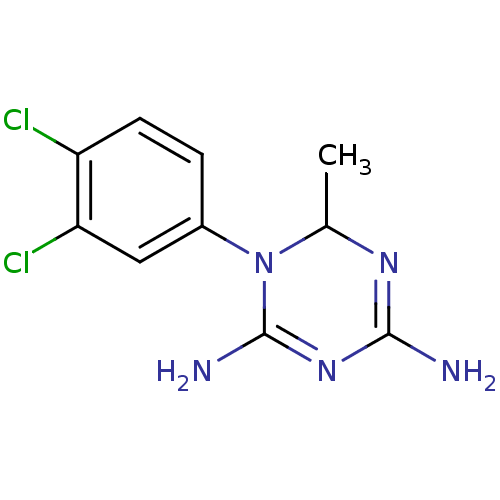
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Yuthavong, Y; Vilaivan, T; Chareonsethakul, N; Kamchonwongpaisan, S; Sirawaraporn, W; Quarrell, R; Lowe, G Development of a lead inhibitor for the A16V+S108T mutant of dihydrofolate reductase from the cycloguanil-resistant strain (T9/94) of Plasmodium falciparum. J Med Chem43:2738-44 (2000) [PubMed]
Yuthavong, Y; Vilaivan, T; Chareonsethakul, N; Kamchonwongpaisan, S; Sirawaraporn, W; Quarrell, R; Lowe, G Development of a lead inhibitor for the A16V+S108T mutant of dihydrofolate reductase from the cycloguanil-resistant strain (T9/94) of Plasmodium falciparum. J Med Chem43:2738-44 (2000) [PubMed]