| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Thymidine kinase |
|---|
| Ligand | BDBM50101064 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEBML_210614 |
|---|
| IC50 | 1.4±n/a nM |
|---|
| Citation |  Martin, JA; Lambert, RW; Merrett, JH; Parkes, KE; Thomas, GJ; Baker, SJ; Bushnell, DJ; Cansfield, JE; Dunsdon, SJ; Freeman, AC; Hopkins, RA; Johns, IR; Keech, E; Simmonite, H; Walmsley, A; Wong Kai-In, P; Holland, M Nucleoside analogues as highly potent and selective inhibitors of herpes simplex virus thymidine kinase. Bioorg Med Chem Lett11:1655-8 (2001) [PubMed] Martin, JA; Lambert, RW; Merrett, JH; Parkes, KE; Thomas, GJ; Baker, SJ; Bushnell, DJ; Cansfield, JE; Dunsdon, SJ; Freeman, AC; Hopkins, RA; Johns, IR; Keech, E; Simmonite, H; Walmsley, A; Wong Kai-In, P; Holland, M Nucleoside analogues as highly potent and selective inhibitors of herpes simplex virus thymidine kinase. Bioorg Med Chem Lett11:1655-8 (2001) [PubMed] |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Thymidine kinase |
|---|
| Name: | Thymidine kinase |
|---|
| Synonyms: | n/a |
|---|
| Type: | PROTEIN |
|---|
| Mol. Mass.: | 40396.73 |
|---|
| Organism: | Human herpesvirus 2 |
|---|
| Description: | ChEMBL_474155 |
|---|
| Residue: | 376 |
|---|
| Sequence: | MASHAGQQHAPAFGQAARASGPTDGRAASRPSHRQGASGARGDPELPTLLRVYIDGPHGV
GKTTTSAQLMEALGPRDNIVYVPEPMTYWQVLGASETLTNIYNTQHRLDRGEISAGEAAV
VMTSAQITMSTPYAATDAVLAPHIGGEAVGPQAPPPALTLVFDRHPIASLLCYPAARYLM
GSMTPQAVLAFVALMPPTAPGTNLVLGVLPEAEHADRLARRQRPGERLDLAMLSAIRRVY
DLLANTVRYLQRGGRWREDWGRLTGVAAATPRPDPEDGAGSLPRIEDTLFALFRVPELLA
PNGDLYHIFAWVLDVLADRLLPMHLFVLDYDQSPVGCRDALLRLTAGMIPTRVTTAGSIA
EIRDLARTFAREVGGV
|
|
|
|---|
| BDBM50101064 |
|---|
| n/a |
|---|
| Name | BDBM50101064 |
|---|
| Synonyms: | 9H-Thioxanthene-9-carboxylic acid [(2R,3S,5R)-5-(5-ethyl-2,4-dioxo-3,4-dihydro-2H-pyrimidin-1-yl)-3-hydroxy-tetrahydro-furan-2-ylmethyl]-amide | CHEMBL288569 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C25H25N3O5S |
|---|
| Mol. Mass. | 479.548 |
|---|
| SMILES | CCc1cn([C@H]2C[C@H](O)[C@@H](CNC(=O)C3c4ccccc4Sc4ccccc34)O2)c(=O)[nH]c1=O |
|---|
| Structure | 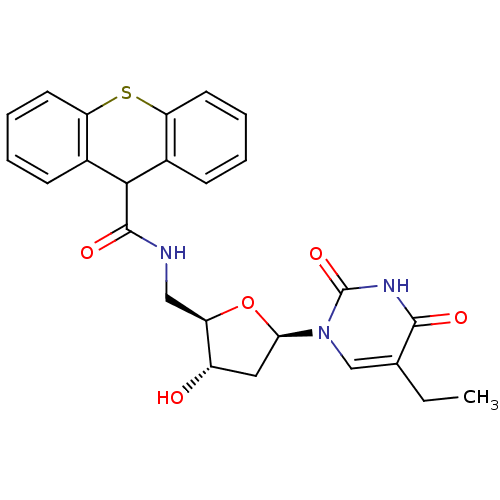
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Martin, JA; Lambert, RW; Merrett, JH; Parkes, KE; Thomas, GJ; Baker, SJ; Bushnell, DJ; Cansfield, JE; Dunsdon, SJ; Freeman, AC; Hopkins, RA; Johns, IR; Keech, E; Simmonite, H; Walmsley, A; Wong Kai-In, P; Holland, M Nucleoside analogues as highly potent and selective inhibitors of herpes simplex virus thymidine kinase. Bioorg Med Chem Lett11:1655-8 (2001) [PubMed]
Martin, JA; Lambert, RW; Merrett, JH; Parkes, KE; Thomas, GJ; Baker, SJ; Bushnell, DJ; Cansfield, JE; Dunsdon, SJ; Freeman, AC; Hopkins, RA; Johns, IR; Keech, E; Simmonite, H; Walmsley, A; Wong Kai-In, P; Holland, M Nucleoside analogues as highly potent and selective inhibitors of herpes simplex virus thymidine kinase. Bioorg Med Chem Lett11:1655-8 (2001) [PubMed]