| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Endothelin-1 receptor |
|---|
| Ligand | BDBM50105003 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_65802 (CHEMBL679705) |
|---|
| IC50 | 0.37±n/a nM |
|---|
| Citation |  Morimoto, H; Shimadzu, H; Kushiyama, E; Kawanishi, H; Hosaka, T; Kawase, Y; Yasuda, K; Kikkawa, K; Yamauchi-Kohno, R; Yamada, K Potent and selective ET-A antagonists. 1. Syntheses and structure-activity relationships of N-(6-(2-(aryloxy)ethoxy)-4-pyrimidinyl)sulfonamide derivatives. J Med Chem44:3355-68 (2001) [PubMed] Morimoto, H; Shimadzu, H; Kushiyama, E; Kawanishi, H; Hosaka, T; Kawase, Y; Yasuda, K; Kikkawa, K; Yamauchi-Kohno, R; Yamada, K Potent and selective ET-A antagonists. 1. Syntheses and structure-activity relationships of N-(6-(2-(aryloxy)ethoxy)-4-pyrimidinyl)sulfonamide derivatives. J Med Chem44:3355-68 (2001) [PubMed] |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Endothelin-1 receptor |
|---|
| Name: | Endothelin-1 receptor |
|---|
| Synonyms: | EDNRA | EDNRA_PIG | Endothelin receptor ET-A |
|---|
| Type: | PROTEIN |
|---|
| Mol. Mass.: | 48707.29 |
|---|
| Organism: | Sus scrofa |
|---|
| Description: | ChEMBL_65803 |
|---|
| Residue: | 427 |
|---|
| Sequence: | METFCFRVSFWVALLGCVISDNPESHSTNLSTHVDDFTTFRGTEFSLVVTTHRPTNLALP
SNGSMHNYCPQQTKITSAFKYINTVISCTIFIVGMVGNATLLRIIYQNKCMRNGPNALIA
SLALGDLIYVVIDLPINVFKLLAGRWPFENHDFGVFLCKLFPFLQKSSVGITVLNLCALS
VDRYRAVASWSRVQGIGIPLVTAIEIVSIWILSFILAIPEAIGFVMVPFEYKGEEHKTCM
LNATSKFMEFYQDVKDWWLFGFYFCMPLVCTAIFYTLMTCEMLNRRNGSLRIALSEHLKQ
RREVAKTVFCLVVIFALCWFPLHLSRILKKTVYDEMDKNRCELLSFLLLMDYIGINLATM
NSCINPIALYFVSKKFKNCFQSCLCCCCYQSKSLMTSVPMNGTSIQWKNHEQNNHNTERS
SHKDSIN
|
|
|
|---|
| BDBM50105003 |
|---|
| n/a |
|---|
| Name | BDBM50105003 |
|---|
| Synonyms: | 4-tert-Butyl-N-{5-(2-methoxy-phenoxy)-6-[2-(5-methylsulfanyl-pyrimidin-2-yloxy)-ethoxy]-pyrimidin-4-yl}-benzenesulfonamide | CHEMBL114660 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C28H31N5O6S2 |
|---|
| Mol. Mass. | 597.706 |
|---|
| SMILES | COc1ccccc1Oc1c(NS(=O)(=O)c2ccc(cc2)C(C)(C)C)ncnc1OCCOc1ncc(SC)cn1 |
|---|
| Structure | 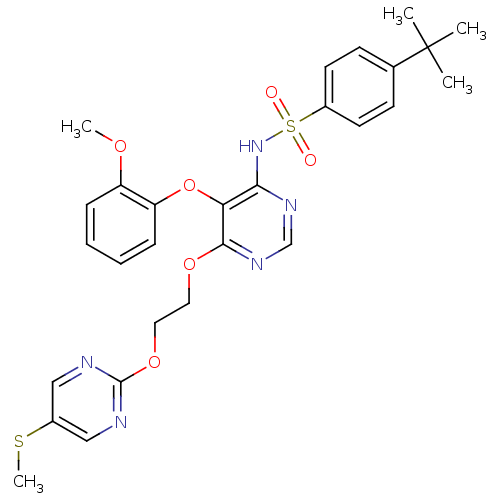
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Morimoto, H; Shimadzu, H; Kushiyama, E; Kawanishi, H; Hosaka, T; Kawase, Y; Yasuda, K; Kikkawa, K; Yamauchi-Kohno, R; Yamada, K Potent and selective ET-A antagonists. 1. Syntheses and structure-activity relationships of N-(6-(2-(aryloxy)ethoxy)-4-pyrimidinyl)sulfonamide derivatives. J Med Chem44:3355-68 (2001) [PubMed]
Morimoto, H; Shimadzu, H; Kushiyama, E; Kawanishi, H; Hosaka, T; Kawase, Y; Yasuda, K; Kikkawa, K; Yamauchi-Kohno, R; Yamada, K Potent and selective ET-A antagonists. 1. Syntheses and structure-activity relationships of N-(6-(2-(aryloxy)ethoxy)-4-pyrimidinyl)sulfonamide derivatives. J Med Chem44:3355-68 (2001) [PubMed]