| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Prostaglandin E2 receptor EP3 subtype |
|---|
| Ligand | BDBM50035622 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_158316 (CHEMBL764942) |
|---|
| IC50 | 110±n/a nM |
|---|
| Citation |  Soper, DL; Milbank, JB; Mieling, GE; Dirr, MJ; Kende, AS; Cooper, R; Jee, WS; Yao, W; Chen, JL; Bodman, M; Lundy, MW; De, B; Stella, ME; Ebetino, FH; Wang, Y; deLong, MA; Wos, JA Synthesis and biological evaluation of prostaglandin-F alkylphosphinic acid derivatives as bone anabolic agents for the treatment of osteoporosis. J Med Chem44:4157-69 (2001) [PubMed] Soper, DL; Milbank, JB; Mieling, GE; Dirr, MJ; Kende, AS; Cooper, R; Jee, WS; Yao, W; Chen, JL; Bodman, M; Lundy, MW; De, B; Stella, ME; Ebetino, FH; Wang, Y; deLong, MA; Wos, JA Synthesis and biological evaluation of prostaglandin-F alkylphosphinic acid derivatives as bone anabolic agents for the treatment of osteoporosis. J Med Chem44:4157-69 (2001) [PubMed] |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Prostaglandin E2 receptor EP3 subtype |
|---|
| Name: | Prostaglandin E2 receptor EP3 subtype |
|---|
| Synonyms: | PE2R3_HUMAN | PGE receptor, EP3 subtype | PGE2-R | PTGER3 | Prostaglandin E2 receptor | Prostaglandin E2 receptor EP3 subtype | Prostaglandin E2 receptor EP3 subtype (EP3) | Prostaglandin E2 receptor EP3A subtype (EP3A) | Prostaglandin E2 receptor EP3D subtype (EP3D) | Prostanoid EP3 receptor |
|---|
| Type: | Enzyme |
|---|
| Mol. Mass.: | 43335.03 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | P43115 |
|---|
| Residue: | 390 |
|---|
| Sequence: | MKETRGYGGDAPFCTRLNHSYTGMWAPERSAEARGNLTRPPGSGEDCGSVSVAFPITMLL
TGFVGNALAMLLVSRSYRRRESKRKKSFLLCIGWLALTDLVGQLLTTPVVIVVYLSKQRW
EHIDPSGRLCTFFGLTMTVFGLSSLFIASAMAVERALAIRAPHWYASHMKTRATRAVLLG
VWLAVLAFALLPVLGVGQYTVQWPGTWCFISTGRGGNGTSSSHNWGNLFFASAFAFLGLL
ALTVTFSCNLATIKALVSRCRAKATASQSSAQWGRITTETAIQLMGIMCVLSVCWSPLLI
MMLKMIFNQTSVEHCKTHTEKQKECNFFLIAVRLASLNQILDPWVYLLLRKILLRKFCQI
RYHTNNYASSSTSLPCQCSSTLMWSDHLER
|
|
|
|---|
| BDBM50035622 |
|---|
| n/a |
|---|
| Name | BDBM50035622 |
|---|
| Synonyms: | (5Z,13E,15S)-9alpha,11alpha,15-trihydroxyprosta-5,13-dien-1-oic acid | CHEMBL815 | DINOPROST | PGF2alpha | l-PGF2-alpha | l-Prostaglandin F2-alpha | prostaglandin F2alpha |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C20H34O5 |
|---|
| Mol. Mass. | 354.481 |
|---|
| SMILES | CCCCC[C@H](O)\C=C\[C@H]1[C@H](O)C[C@H](O)[C@@H]1C\C=C/CCCC(O)=O |r| |
|---|
| Structure | 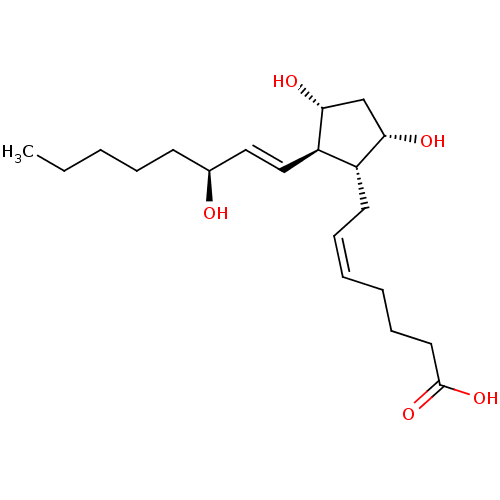
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Soper, DL; Milbank, JB; Mieling, GE; Dirr, MJ; Kende, AS; Cooper, R; Jee, WS; Yao, W; Chen, JL; Bodman, M; Lundy, MW; De, B; Stella, ME; Ebetino, FH; Wang, Y; deLong, MA; Wos, JA Synthesis and biological evaluation of prostaglandin-F alkylphosphinic acid derivatives as bone anabolic agents for the treatment of osteoporosis. J Med Chem44:4157-69 (2001) [PubMed]
Soper, DL; Milbank, JB; Mieling, GE; Dirr, MJ; Kende, AS; Cooper, R; Jee, WS; Yao, W; Chen, JL; Bodman, M; Lundy, MW; De, B; Stella, ME; Ebetino, FH; Wang, Y; deLong, MA; Wos, JA Synthesis and biological evaluation of prostaglandin-F alkylphosphinic acid derivatives as bone anabolic agents for the treatment of osteoporosis. J Med Chem44:4157-69 (2001) [PubMed]