| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Endothelin receptor type B |
|---|
| Ligand | BDBM50107562 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEBML_64041 |
|---|
| IC50 | >1±n/a nM |
|---|
| Citation |  Morimoto, H; Shimadzu, H; Hosaka, T; Kawase, Y; Yasuda, K; Kikkawa, K; Yamauchi-Kohno, R; Yamada, K Modifications and structure-activity relationships at the 2-position of 4-sulfonamidopyrimidine derivatives as potent endothelin antagonists. Bioorg Med Chem Lett12:81-4 (2001) [PubMed] Morimoto, H; Shimadzu, H; Hosaka, T; Kawase, Y; Yasuda, K; Kikkawa, K; Yamauchi-Kohno, R; Yamada, K Modifications and structure-activity relationships at the 2-position of 4-sulfonamidopyrimidine derivatives as potent endothelin antagonists. Bioorg Med Chem Lett12:81-4 (2001) [PubMed] |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Endothelin receptor type B |
|---|
| Name: | Endothelin receptor type B |
|---|
| Synonyms: | EDNRB_RAT | ENDOTHELIN B | ET-B | Ednrb | Endothelin receptor | Endothelin receptor non-selective type |
|---|
| Type: | Enzyme Catalytic Domain |
|---|
| Mol. Mass.: | 49483.43 |
|---|
| Organism: | RAT |
|---|
| Description: | ENDOTHELIN B EDNRB RAT::P21451 |
|---|
| Residue: | 442 |
|---|
| Sequence: | MQSSASRCGRALVALLLACGLLGVWGEKRGFPPAQATPSLLGTKEVMTPPTKTSWTRGSN
SSLMRSSAPAEVTKGGRVAGVPPRSFPPPCQRKIEINKTFKYINTIVSCLVFVLGIIGNS
TLLRIIYKNKCMRNGPNILIASLALGDLLHIIIDIPINAYKLLAGDWPFGAEMCKLVPFI
QKASVGITVLSLCALSIDRYRAVASWSRIKGIGVPKWTAVEIVLIWVVSVVLAVPEAIGF
DVITSDYKGKPLRVCMLNPFQKTAFMQFYKTAKDWWLFSFYFCLPLAITAIFYTLMTCEM
LRKKSGMQIALNDHLKQRREVAKTVFCLVLVFALCWLPLHLSRILKLTLYDQSNPQRCEL
LSFLLVLDYIGINMASLNSCINPIALYLVSKRFKNCFKSCLCCWCQTFEEKQSLEEKQSC
LKFKANDHGYDNFRSSNKYSSS
|
|
|
|---|
| BDBM50107562 |
|---|
| n/a |
|---|
| Name | BDBM50107562 |
|---|
| Synonyms: | CHEMBL168119 | N-[6-[2-(5-Bromo-pyrimidin-2-yloxy)-ethoxy]-2-(4-hydroxy-piperidin-1-yl)-5-(2-methoxy-phenoxy)-pyrimidin-4-yl]-4-tert-butyl-benzenesulfonamide |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C32H37BrN6O7S |
|---|
| Mol. Mass. | 729.641 |
|---|
| SMILES | COc1ccccc1Oc1c(NS(=O)(=O)c2ccc(cc2)C(C)(C)C)nc(nc1OCCOc1ncc(Br)cn1)N1CCC(O)CC1 |
|---|
| Structure | 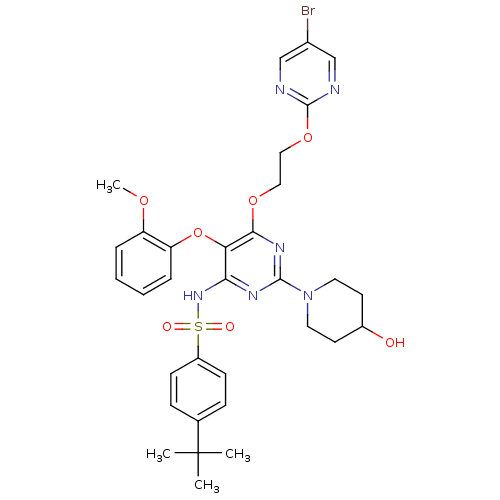
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Morimoto, H; Shimadzu, H; Hosaka, T; Kawase, Y; Yasuda, K; Kikkawa, K; Yamauchi-Kohno, R; Yamada, K Modifications and structure-activity relationships at the 2-position of 4-sulfonamidopyrimidine derivatives as potent endothelin antagonists. Bioorg Med Chem Lett12:81-4 (2001) [PubMed]
Morimoto, H; Shimadzu, H; Hosaka, T; Kawase, Y; Yasuda, K; Kikkawa, K; Yamauchi-Kohno, R; Yamada, K Modifications and structure-activity relationships at the 2-position of 4-sulfonamidopyrimidine derivatives as potent endothelin antagonists. Bioorg Med Chem Lett12:81-4 (2001) [PubMed]