| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Genome polyprotein |
|---|
| Ligand | BDBM50110153 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEBML_143654 |
|---|
| IC50 | 300±n/a nM |
|---|
| Citation |  Colarusso, S; Gerlach, B; Koch, U; Muraglia, E; Conte, I; Stansfield, I; Matassa, VG; Narjes, F Evolution, synthesis and SAR of tripeptide alpha-ketoacid inhibitors of the hepatitis C virus NS3/NS4A serine protease. Bioorg Med Chem Lett12:705-8 (2002) [PubMed] Colarusso, S; Gerlach, B; Koch, U; Muraglia, E; Conte, I; Stansfield, I; Matassa, VG; Narjes, F Evolution, synthesis and SAR of tripeptide alpha-ketoacid inhibitors of the hepatitis C virus NS3/NS4A serine protease. Bioorg Med Chem Lett12:705-8 (2002) [PubMed] |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Genome polyprotein |
|---|
| Name: | Genome polyprotein |
|---|
| Synonyms: | Genome polyprotein | Genome polyprotein (NS3-NS4A) | Hepatitis C virus polyprotein | POLG_HCV1 | RNA-dependent RNA polymerase (NS5B) |
|---|
| Type: | Protein |
|---|
| Mol. Mass.: | 327266.82 |
|---|
| Organism: | Hepatitis C virus (HCV) |
|---|
| Description: | P26664 |
|---|
| Residue: | 3011 |
|---|
| Sequence: | MSTNPKPQKKNKRNTNRRPQDVKFPGGGQIVGGVYLLPRRGPRLGVRATRKTSERSQPRG
RRQPIPKARRPEGRTWAQPGYPWPLYGNEGCGWAGWLLSPRGSRPSWGPTDPRRRSRNLG
KVIDTLTCGFADLMGYIPLVGAPLGGAARALAHGVRVLEDGVNYATGNLPGCSFSIFLLA
LLSCLTVPASAYQVRNSTGLYHVTNDCPNSSIVYEAADAILHTPGCVPCVREGNASRCWV
AMTPTVATRDGKLPATQLRRHIDLLVGSATLCSALYVGDLCGSVFLVGQLFTFSPRRHWT
TQGCNCSIYPGHITGHRMAWDMMMNWSPTTALVMAQLLRIPQAILDMIAGAHWGVLAGIA
YFSMVGNWAKVLVVLLLFAGVDAETHVTGGSAGHTVSGFVSLLAPGAKQNVQLINTNGSW
HLNSTALNCNDSLNTGWLAGLFYHHKFNSSGCPERLASCRPLTDFDQGWGPISYANGSGP
DQRPYCWHYPPKPCGIVPAKSVCGPVYCFTPSPVVVGTTDRSGAPTYSWGENDTDVFVLN
NTRPPLGNWFGCTWMNSTGFTKVCGAPPCVIGGAGNNTLHCPTDCFRKHPDATYSRCGSG
PWITPRCLVDYPYRLWHYPCTINYTIFKIRMYVGGVEHRLEAACNWTRGERCDLEDRDRS
ELSPLLLTTTQWQVLPCSFTTLPALSTGLIHLHQNIVDVQYLYGVGSSIASWAIKWEYVV
LLFLLLADARVCSCLWMMLLISQAEAALENLVILNAASLAGTHGLVSFLVFFCFAWYLKG
KWVPGAVYTFYGMWPLLLLLLALPQRAYALDTEVAASCGGVVLVGLMALTLSPYYKRYIS
WCLWWLQYFLTRVEAQLHVWIPPLNVRGGRDAVILLMCAVHPTLVFDITKLLLAVFGPLW
ILQASLLKVPYFVRVQGLLRFCALARKMIGGHYVQMVIIKLGALTGTYVYNHLTPLRDWA
HNGLRDLAVAVEPVVFSQMETKLITWGADTAACGDIINGLPVSARRGREILLGPADGMVS
KGWRLLAPITAYAQQTRGLLGCIITSLTGRDKNQVEGEVQIVSTAAQTFLATCINGVCWT
VYHGAGTRTIASPKGPVIQMYTNVDQDLVGWPAPQGSRSLTPCTCGSSDLYLVTRHADVI
PVRRRGDSRGSLLSPRPISYLKGSSGGPLLCPAGHAVGIFRAAVCTRGVAKAVDFIPVEN
LETTMRSPVFTDNSSPPVVPQSFQVAHLHAPTGSGKSTKVPAAYAAQGYKVLVLNPSVAA
TLGFGAYMSKAHGIDPNIRTGVRTITTGSPITYSTYGKFLADGGCSGGAYDIIICDECHS
TDATSILGIGTVLDQAETAGARLVVLATATPPGSVTVPHPNIEEVALSTTGEIPFYGKAI
PLEVIKGGRHLIFCHSKKKCDELAAKLVALGINAVAYYRGLDVSVIPTSGDVVVVATDAL
MTGYTGDFDSVIDCNTCVTQTVDFSLDPTFTIETITLPQDAVSRTQRRGRTGRGKPGIYR
FVAPGERPSGMFDSSVLCECYDAGCAWYELTPAETTVRLRAYMNTPGLPVCQDHLEFWEG
VFTGLTHIDAHFLSQTKQSGENLPYLVAYQATVCARAQAPPPSWDQMWKCLIRLKPTLHG
PTPLLYRLGAVQNEITLTHPVTKYIMTCMSADLEVVTSTWVLVGGVLAALAAYCLSTGCV
VIVGRVVLSGKPAIIPDREVLYREFDEMEECSQHLPYIEQGMMLAEQFKQKALGLLQTAS
RQAEVIAPAVQTNWQKLETFWAKHMWNFISGIQYLAGLSTLPGNPAIASLMAFTAAVTSP
LTTSQTLLFNILGGWVAAQLAAPGAATAFVGAGLAGAAIGSVGLGKVLIDILAGYGAGVA
GALVAFKIMSGEVPSTEDLVNLLPAILSPGALVVGVVCAAILRRHVGPGEGAVQWMNRLI
AFASRGNHVSPTHYVPESDAAARVTAILSSLTVTQLLRRLHQWISSECTTPCSGSWLRDI
WDWICEVLSDFKTWLKAKLMPQLPGIPFVSCQRGYKGVWRVDGIMHTRCHCGAEITGHVK
NGTMRIVGPRTCRNMWSGTFPINAYTTGPCTPLPAPNYTFALWRVSAEEYVEIRQVGDFH
YVTGMTTDNLKCPCQVPSPEFFTELDGVRLHRFAPPCKPLLREEVSFRVGLHEYPVGSQL
PCEPEPDVAVLTSMLTDPSHITAEAAGRRLARGSPPSVASSSASQLSAPSLKATCTANHD
SPDAELIEANLLWRQEMGGNITRVESENKVVILDSFDPLVAEEDEREISVPAEILRKSRR
FAQALPVWARPDYNPPLVETWKKPDYEPPVVHGCPLPPPKSPPVPPPRKKRTVVLTESTL
STALAELATRSFGSSSTSGITGDNTTTSSEPAPSGCPPDSDAESYSSMPPLEGEPGDPDL
SDGSWSTVSSEANAEDVVCCSMSYSWTGALVTPCAAEEQKLPINALSNSLLRHHNLVYST
TSRSACQRQKKVTFDRLQVLDSHYQDVLKEVKAAASKVKANLLSVEEACSLTPPHSAKSK
FGYGAKDVRCHARKAVTHINSVWKDLLEDNVTPIDTTIMAKNEVFCVQPEKGGRKPARLI
VFPDLGVRVCEKMALYDVVTKLPLAVMGSSYGFQYSPGQRVEFLVQAWKSKKTPMGFSYD
TRCFDSTVTESDIRTEEAIYQCCDLDPQARVAIKSLTERLYVGGPLTNSRGENCGYRRCR
ASGVLTTSCGNTLTCYIKARAACRAAGLQDCTMLVCGDDLVVICESAGVQEDAASLRAFT
EAMTRYSAPPGDPPQPEYDLELITSCSSNVSVAHDGAGKRVYYLTRDPTTPLARAAWETA
RHTPVNSWLGNIIMFAPTLWARMILMTHFFSVLIARDQLEQALDCEIYGACYSIEPLDLP
PIIQRLHGLSAFSLHSYSPGEINRVAACLRKLGVPPLRAWRHRARSVRARLLARGGRAAI
CGKYLFNWAVRTKLKLTPIAAAGQLDLSGWFTAGYSGGDIYHSVSHARPRWIWFCLLLLA
AGVGIYLLPNR
|
|
|
|---|
| BDBM50110153 |
|---|
| n/a |
|---|
| Name | BDBM50110153 |
|---|
| Synonyms: | (S)-3-[(S)-2-((S)-2-tert-Butoxycarbonylamino-4-carboxy-butyrylamino)-4,4-difluoro-butyrylamino]-5,5-difluoro-2-oxo-pentanoic acid | CHEMBL160018 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C19H27F4N3O9 |
|---|
| Mol. Mass. | 517.426 |
|---|
| SMILES | CC(C)(C)OC(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CC(F)F)C(=O)N[C@@H](CC(F)F)C(=O)C(O)=O |
|---|
| Structure | 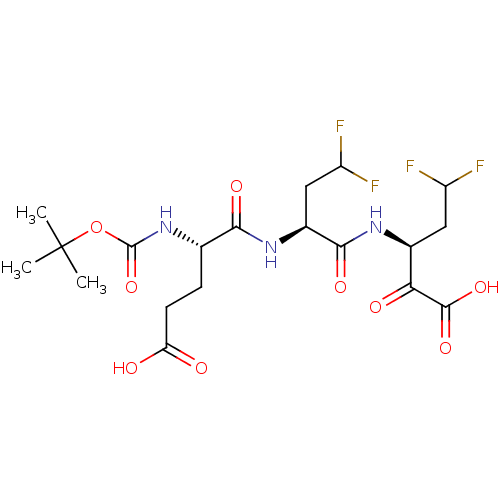
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Colarusso, S; Gerlach, B; Koch, U; Muraglia, E; Conte, I; Stansfield, I; Matassa, VG; Narjes, F Evolution, synthesis and SAR of tripeptide alpha-ketoacid inhibitors of the hepatitis C virus NS3/NS4A serine protease. Bioorg Med Chem Lett12:705-8 (2002) [PubMed]
Colarusso, S; Gerlach, B; Koch, U; Muraglia, E; Conte, I; Stansfield, I; Matassa, VG; Narjes, F Evolution, synthesis and SAR of tripeptide alpha-ketoacid inhibitors of the hepatitis C virus NS3/NS4A serine protease. Bioorg Med Chem Lett12:705-8 (2002) [PubMed]