| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Receptor-type tyrosine-protein kinase FLT3 |
|---|
| Ligand | BDBM50115298 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_68356 (CHEMBL679498) |
|---|
| IC50 | 11400±n/a nM |
|---|
| Citation |  Matsuno, K; Ichimura, M; Nakajima, T; Tahara, K; Fujiwara, S; Kase, H; Ushiki, J; Giese, NA; Pandey, A; Scarborough, RM; Lokker, NA; Yu, JC; Irie, J; Tsukuda, E; Ide, S; Oda, S; Nomoto, Y Potent and selective inhibitors of platelet-derived growth factor receptor phosphorylation. 1. Synthesis, structure-activity relationship, and biological effects of a new class of quinazoline derivatives. J Med Chem45:3057-66 (2002) [PubMed] Matsuno, K; Ichimura, M; Nakajima, T; Tahara, K; Fujiwara, S; Kase, H; Ushiki, J; Giese, NA; Pandey, A; Scarborough, RM; Lokker, NA; Yu, JC; Irie, J; Tsukuda, E; Ide, S; Oda, S; Nomoto, Y Potent and selective inhibitors of platelet-derived growth factor receptor phosphorylation. 1. Synthesis, structure-activity relationship, and biological effects of a new class of quinazoline derivatives. J Med Chem45:3057-66 (2002) [PubMed] |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Receptor-type tyrosine-protein kinase FLT3 |
|---|
| Name: | Receptor-type tyrosine-protein kinase FLT3 |
|---|
| Synonyms: | CD135 | CD_antigen: CD135 | FL cytokine receptor | FLK-2 | FLK2 | FLT-3 | FLT3 | FLT3_HUMAN | Fetal liver kinase-2 | Fms-like tyrosine kinase 3 | Fms-like tyrosine kinase 3 (Flt-3) | Fms-related tyrosine kinase 3 | STK-1 | STK1 | Stem cell tyrosine kinase 1 |
|---|
| Type: | Enzyme |
|---|
| Mol. Mass.: | 112888.62 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | P36888 |
|---|
| Residue: | 993 |
|---|
| Sequence: | MPALARDGGQLPLLVVFSAMIFGTITNQDLPVIKCVLINHKNNDSSVGKSSSYPMVSESP
EDLGCALRPQSSGTVYEAAAVEVDVSASITLQVLVDAPGNISCLWVFKHSSLNCQPHFDL
QNRGVVSMVILKMTETQAGEYLLFIQSEATNYTILFTVSIRNTLLYTLRRPYFRKMENQD
ALVCISESVPEPIVEWVLCDSQGESCKEESPAVVKKEEKVLHELFGTDIRCCARNELGRE
CTRLFTIDLNQTPQTTLPQLFLKVGEPLWIRCKAVHVNHGFGLTWELENKALEEGNYFEM
STYSTNRTMIRILFAFVSSVARNDTGYYTCSSSKHPSQSALVTIVEKGFINATNSSEDYE
IDQYEEFCFSVRFKAYPQIRCTWTFSRKSFPCEQKGLDNGYSISKFCNHKHQPGEYIFHA
ENDDAQFTKMFTLNIRRKPQVLAEASASQASCFSDGYPLPSWTWKKCSDKSPNCTEEITE
GVWNRKANRKVFGQWVSSSTLNMSEAIKGFLVKCCAYNSLGTSCETILLNSPGPFPFIQD
NISFYATIGVCLLFIVVLTLLICHKYKKQFRYESQLQMVQVTGSSDNEYFYVDFREYEYD
LKWEFPRENLEFGKVLGSGAFGKVMNATAYGISKTGVSIQVAVKMLKEKADSSEREALMS
ELKMMTQLGSHENIVNLLGACTLSGPIYLIFEYCCYGDLLNYLRSKREKFHRTWTEIFKE
HNFSFYPTFQSHPNSSMPGSREVQIHPDSDQISGLHGNSFHSEDEIEYENQKRLEEEEDL
NVLTFEDLLCFAYQVAKGMEFLEFKSCVHRDLAARNVLVTHGKVVKICDFGLARDIMSDS
NYVVRGNARLPVKWMAPESLFEGIYTIKSDVWSYGILLWEIFSLGVNPYPGIPVDANFYK
LIQNGFKMDQPFYATEEIYIIMQSCWAFDSRKRPSFPNLTSFLGCQLADAEEAMYQNVDG
RVSECPHTYQNRRPFSREMDLGLLSPQAQVEDS
|
|
|
|---|
| BDBM50115298 |
|---|
| n/a |
|---|
| Name | BDBM50115298 |
|---|
| Synonyms: | 4-(6,7-Dimethoxy-quinazolin-4-yl)-piperazine-1-carboxylic acid (4-bromo-phenyl)-amide | CHEMBL323336 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C21H22BrN5O3 |
|---|
| Mol. Mass. | 472.335 |
|---|
| SMILES | COc1cc2ncnc(N3CCN(CC3)C(=O)Nc3ccc(Br)cc3)c2cc1OC |
|---|
| Structure | 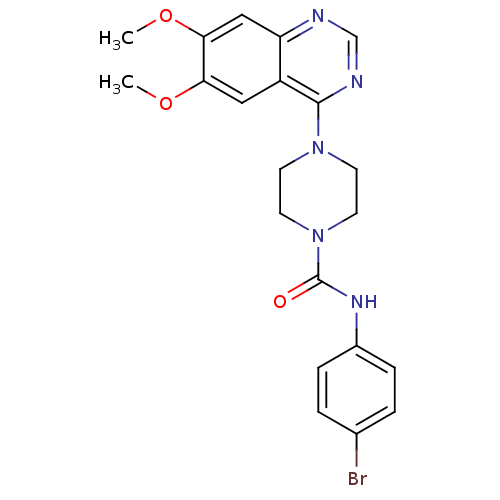
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Matsuno, K; Ichimura, M; Nakajima, T; Tahara, K; Fujiwara, S; Kase, H; Ushiki, J; Giese, NA; Pandey, A; Scarborough, RM; Lokker, NA; Yu, JC; Irie, J; Tsukuda, E; Ide, S; Oda, S; Nomoto, Y Potent and selective inhibitors of platelet-derived growth factor receptor phosphorylation. 1. Synthesis, structure-activity relationship, and biological effects of a new class of quinazoline derivatives. J Med Chem45:3057-66 (2002) [PubMed]
Matsuno, K; Ichimura, M; Nakajima, T; Tahara, K; Fujiwara, S; Kase, H; Ushiki, J; Giese, NA; Pandey, A; Scarborough, RM; Lokker, NA; Yu, JC; Irie, J; Tsukuda, E; Ide, S; Oda, S; Nomoto, Y Potent and selective inhibitors of platelet-derived growth factor receptor phosphorylation. 1. Synthesis, structure-activity relationship, and biological effects of a new class of quinazoline derivatives. J Med Chem45:3057-66 (2002) [PubMed]