| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Mycothiol S-conjugate amidase |
|---|
| Ligand | BDBM50117079 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEBML_140808 |
|---|
| IC50 | 37000±n/a nM |
|---|
| Citation |  Nicholas, GM; Eckman, LL; Ray, S; Hughes, RO; Pfefferkorn, JA; Barluenga, S; Nicolaou, KC; Bewley, CA Bromotyrosine-derived natural and synthetic products as inhibitors of mycothiol-S-conjugate amidase. Bioorg Med Chem Lett12:2487-90 (2002) [PubMed] Nicholas, GM; Eckman, LL; Ray, S; Hughes, RO; Pfefferkorn, JA; Barluenga, S; Nicolaou, KC; Bewley, CA Bromotyrosine-derived natural and synthetic products as inhibitors of mycothiol-S-conjugate amidase. Bioorg Med Chem Lett12:2487-90 (2002) [PubMed] |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Mycothiol S-conjugate amidase |
|---|
| Name: | Mycothiol S-conjugate amidase |
|---|
| Synonyms: | MCA_MYCTU | mca |
|---|
| Type: | PROTEIN |
|---|
| Mol. Mass.: | 32719.79 |
|---|
| Organism: | Mycobacterium tuberculosis |
|---|
| Description: | ChEMBL_626858 |
|---|
| Residue: | 288 |
|---|
| Sequence: | MSELRLMAVHAHPDDESSKGAATLARYADEGHRVLVVTLTGGERGEILNPAMDLPDVHGR
IAEIRRDEMTKAAEILGVEHTWLGFVDSGLPKGDLPPPLPDDCFARVPLEVSTEALVRVV
REFRPHVMTTYDENGGYPHPDHIRCHQVSVAAYEAAGDFCRFPDAGEPWTVSKLYYVHGF
LRERMQMLQDEFARHGQRGPFEQWLAYWDPDHDFLTSRVTTRVECSKYFSQRDDALRAHA
TQIDPNAEFFAAPLAWQERLWPTEEFELARSRIPARPPETELFAGIEP
|
|
|
|---|
| BDBM50117079 |
|---|
| n/a |
|---|
| Name | BDBM50117079 |
|---|
| Synonyms: | 3-(3-Chloro-4-hydroxy-phenyl)-N-[2-(4-fluoro-phenyldisulfanyl)-ethyl]-2-[(Z)-hydroxyimino]-propionamide | CHEMBL81695 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C17H16ClFN2O3S2 |
|---|
| Mol. Mass. | 414.902 |
|---|
| SMILES | Oc1ccc(CC(N=O)C(=O)NCCSSc2ccc(F)cc2)cc1Cl |
|---|
| Structure | 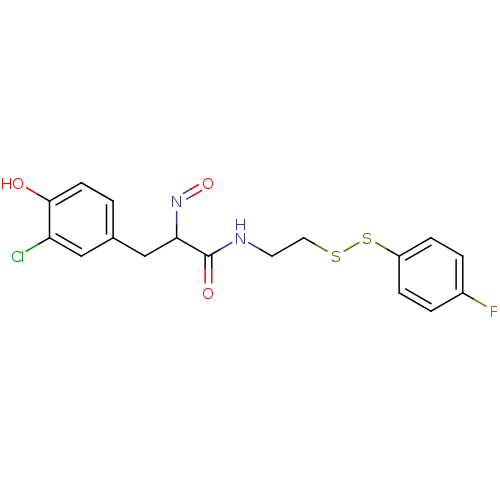
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Nicholas, GM; Eckman, LL; Ray, S; Hughes, RO; Pfefferkorn, JA; Barluenga, S; Nicolaou, KC; Bewley, CA Bromotyrosine-derived natural and synthetic products as inhibitors of mycothiol-S-conjugate amidase. Bioorg Med Chem Lett12:2487-90 (2002) [PubMed]
Nicholas, GM; Eckman, LL; Ray, S; Hughes, RO; Pfefferkorn, JA; Barluenga, S; Nicolaou, KC; Bewley, CA Bromotyrosine-derived natural and synthetic products as inhibitors of mycothiol-S-conjugate amidase. Bioorg Med Chem Lett12:2487-90 (2002) [PubMed]