| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Ribosyldihydronicotinamide dehydrogenase [quinone] |
|---|
| Ligand | BDBM50118457 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_104940 (CHEMBL714719) |
|---|
| Ki | 782±n/a nM |
|---|
| Citation |  Fukatsu, K; Uchikawa, O; Kawada, M; Yamano, T; Yamashita, M; Kato, K; Hirai, K; Hinuma, S; Miyamoto, M; Ohkawa, S Synthesis of a novel series of benzocycloalkene derivatives as melatonin receptor agonists. J Med Chem45:4212-21 (2002) [PubMed] Fukatsu, K; Uchikawa, O; Kawada, M; Yamano, T; Yamashita, M; Kato, K; Hirai, K; Hinuma, S; Miyamoto, M; Ohkawa, S Synthesis of a novel series of benzocycloalkene derivatives as melatonin receptor agonists. J Med Chem45:4212-21 (2002) [PubMed] |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Ribosyldihydronicotinamide dehydrogenase [quinone] |
|---|
| Name: | Ribosyldihydronicotinamide dehydrogenase [quinone] |
|---|
| Synonyms: | Metallothionein-3 | NMOR2 | NQO2 | NQO2_HUMAN | NRH dehydrogenase [quinone] 2 | NRH:quinone oxidoreductase 2 | QR2 | Quinone reductase 2 | Quinone reductase 2 (NQO2) | Ribosyldihydronicotinamide dehydrogenase [quinone] |
|---|
| Type: | Protein |
|---|
| Mol. Mass.: | 25917.25 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | P16083 |
|---|
| Residue: | 231 |
|---|
| Sequence: | MAGKKVLIVYAHQEPKSFNGSLKNVAVDELSRQGCTVTVSDLYAMNLEPRATDKDITGTL
SNPEVFNYGVETHEAYKQRSLASDITDEQKKVREADLVIFQFPLYWFSVPAILKGWMDRV
LCQGFAFDIPGFYDSGLLQGKLALLSVTTGGTAEMYTKTGVNGDSRYFLWPLQHGTLHFC
GFKVLAPQISFAPEIASEEERKGMVAAWSQRLQTIWKEEPIPCTAHWHFGQ
|
|
|
|---|
| BDBM50118457 |
|---|
| n/a |
|---|
| Name | BDBM50118457 |
|---|
| Synonyms: | CHEMBL339840 | N-[2-(5,6-Dimethoxy-indan-1-yl)-ethyl]-acetamide |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C15H21NO3 |
|---|
| Mol. Mass. | 263.3321 |
|---|
| SMILES | COc1cc2CCC(CCNC(C)=O)c2cc1OC |
|---|
| Structure | 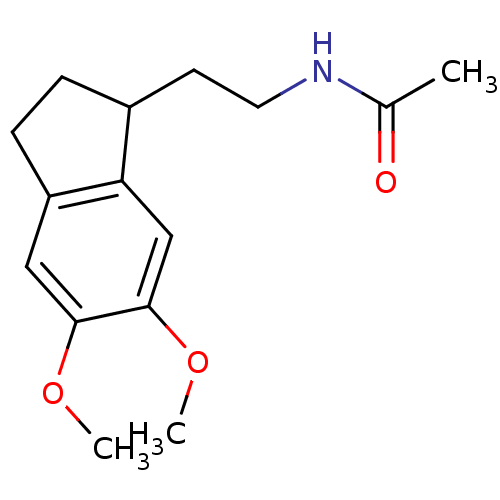
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Fukatsu, K; Uchikawa, O; Kawada, M; Yamano, T; Yamashita, M; Kato, K; Hirai, K; Hinuma, S; Miyamoto, M; Ohkawa, S Synthesis of a novel series of benzocycloalkene derivatives as melatonin receptor agonists. J Med Chem45:4212-21 (2002) [PubMed]
Fukatsu, K; Uchikawa, O; Kawada, M; Yamano, T; Yamashita, M; Kato, K; Hirai, K; Hinuma, S; Miyamoto, M; Ohkawa, S Synthesis of a novel series of benzocycloalkene derivatives as melatonin receptor agonists. J Med Chem45:4212-21 (2002) [PubMed]