| Reaction Details |
|---|
 | Report a problem with these data |
| Target | 72 kDa type IV collagenase |
|---|
| Ligand | BDBM50120695 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_104397 (CHEMBL715000) |
|---|
| Ki | >3000±n/a nM |
|---|
| Citation |  Duan, JJ; Chen, L; Wasserman, ZR; Lu, Z; Liu, RQ; Covington, MB; Qian, M; Hardman, KD; Magolda, RL; Newton, RC; Christ, DD; Wexler, RR; Decicco, CP Discovery of gamma-lactam hydroxamic acids as selective inhibitors of tumor necrosis factor alpha converting enzyme: design, synthesis, and structure-activity relationships. J Med Chem45:4954-7 (2002) [PubMed] Duan, JJ; Chen, L; Wasserman, ZR; Lu, Z; Liu, RQ; Covington, MB; Qian, M; Hardman, KD; Magolda, RL; Newton, RC; Christ, DD; Wexler, RR; Decicco, CP Discovery of gamma-lactam hydroxamic acids as selective inhibitors of tumor necrosis factor alpha converting enzyme: design, synthesis, and structure-activity relationships. J Med Chem45:4954-7 (2002) [PubMed] |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| 72 kDa type IV collagenase |
|---|
| Name: | 72 kDa type IV collagenase |
|---|
| Synonyms: | 72 kDa gelatinase | 72 kDa type IV collagenase precursor | CLG4A | Gelatinase A | Gelatinase A (MMP-2) | MMP2 | MMP2_HUMAN | Matrix metalloproteinase-2 | Matrix metalloproteinase-2 (MMP 2) | Matrix metalloproteinase-2 (MMP2) | Matrix metalloproteinases 2 (MMP-2) | TBE-1 |
|---|
| Type: | Enzyme |
|---|
| Mol. Mass.: | 73870.36 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | P08253 |
|---|
| Residue: | 660 |
|---|
| Sequence: | MEALMARGALTGPLRALCLLGCLLSHAAAAPSPIIKFPGDVAPKTDKELAVQYLNTFYGC
PKESCNLFVLKDTLKKMQKFFGLPQTGDLDQNTIETMRKPRCGNPDVANYNFFPRKPKWD
KNQITYRIIGYTPDLDPETVDDAFARAFQVWSDVTPLRFSRIHDGEADIMINFGRWEHGD
GYPFDGKDGLLAHAFAPGTGVGGDSHFDDDELWTLGEGQVVRVKYGNADGEYCKFPFLFN
GKEYNSCTDTGRSDGFLWCSTTYNFEKDGKYGFCPHEALFTMGGNAEGQPCKFPFRFQGT
SYDSCTTEGRTDGYRWCGTTEDYDRDKKYGFCPETAMSTVGGNSEGAPCVFPFTFLGNKY
ESCTSAGRSDGKMWCATTANYDDDRKWGFCPDQGYSLFLVAAHEFGHAMGLEHSQDPGAL
MAPIYTYTKNFRLSQDDIKGIQELYGASPDIDLGTGPTPTLGPVTPEICKQDIVFDGIAQ
IRGEIFFFKDRFIWRTVTPRDKPMGPLLVATFWPELPEKIDAVYEAPQEEKAVFFAGNEY
WIYSASTLERGYPKPLTSLGLPPDVQRVDAAFNWSKNKKTYIFAGDKFWRYNEVKKKMDP
GFPKLIADAWNAIPDNLDAVVDLQGGGHSYFFKGAYYLKLENQSLKSVKFGSIKSDWLGC
|
|
|
|---|
| BDBM50120695 |
|---|
| n/a |
|---|
| Name | BDBM50120695 |
|---|
| Synonyms: | 2-{3-[4-(3,5-Dimethoxy-benzyloxy)-phenyl]-3-methyl-2-oxo-pyrrolidin-1-yl}-N-hydroxy-propionamide | CHEMBL143451 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C23H28N2O6 |
|---|
| Mol. Mass. | 428.4782 |
|---|
| SMILES | COc1cc(COc2ccc(cc2)[C@]2(C)CCN([C@H](C)C(=O)NO)C2=O)cc(OC)c1 |
|---|
| Structure | 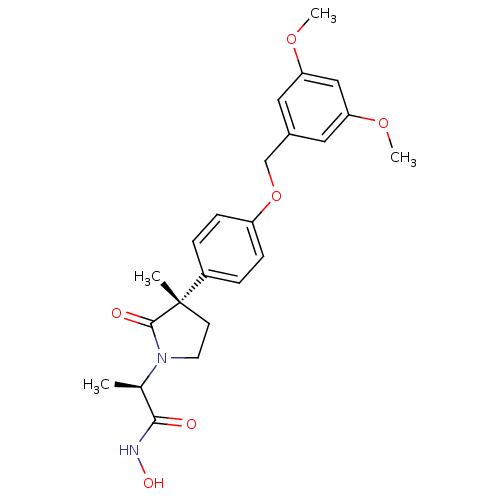
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Duan, JJ; Chen, L; Wasserman, ZR; Lu, Z; Liu, RQ; Covington, MB; Qian, M; Hardman, KD; Magolda, RL; Newton, RC; Christ, DD; Wexler, RR; Decicco, CP Discovery of gamma-lactam hydroxamic acids as selective inhibitors of tumor necrosis factor alpha converting enzyme: design, synthesis, and structure-activity relationships. J Med Chem45:4954-7 (2002) [PubMed]
Duan, JJ; Chen, L; Wasserman, ZR; Lu, Z; Liu, RQ; Covington, MB; Qian, M; Hardman, KD; Magolda, RL; Newton, RC; Christ, DD; Wexler, RR; Decicco, CP Discovery of gamma-lactam hydroxamic acids as selective inhibitors of tumor necrosis factor alpha converting enzyme: design, synthesis, and structure-activity relationships. J Med Chem45:4954-7 (2002) [PubMed]