| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Procathepsin L |
|---|
| Ligand | BDBM50121040 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEBML_48518 |
|---|
| IC50 | 1.7±n/a nM |
|---|
| Citation |  Zhou, NE; Kaleta, J; Purisima, E; Menard, R; Micetich, RG; Singh, R 6-Acylamino-penam derivatives: synthesis and inhibition of cathepsins B, L, K, and S. Bioorg Med Chem Lett12:3417-9 (2002) [PubMed] Zhou, NE; Kaleta, J; Purisima, E; Menard, R; Micetich, RG; Singh, R 6-Acylamino-penam derivatives: synthesis and inhibition of cathepsins B, L, K, and S. Bioorg Med Chem Lett12:3417-9 (2002) [PubMed] |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Procathepsin L |
|---|
| Name: | Procathepsin L |
|---|
| Synonyms: | CATL1_RAT | Cathepsin L | Ctsl | Ctsl1 |
|---|
| Type: | PROTEIN |
|---|
| Mol. Mass.: | 37662.10 |
|---|
| Organism: | Rattus norvegicus |
|---|
| Description: | ChEMBL_48517 |
|---|
| Residue: | 334 |
|---|
| Sequence: | MTPLLLLAVLCLGTALATPKFDQTFNAQWHQWKSTHRRLYGTNEEEWRRAVWEKNMRMIQ
LHNGEYSNGKHGFTMEMNAFGDMTNEEFRQIVNGYRHQKHKKGRLFQEPLMLQIPKTVDW
REKGCVTPVKNQGQCGSCWAFSASGCLEGQMFLKTGKLISLSEQNLVDCSHDQGNQGCNG
GLMDFAFQYIKENGGLDSEESYPYEAKDGSCKYRAEYAVANDTGFVDIPQQEKALMKAVA
TVGPISVAMDASHPSLQFYSSGIYYEPNCSSKDLDHGVLVVGYGYEGTDSNKDKYWLVKN
SWGKEWGMDGYIKIAKDRNNHCGLATAASYPIVN
|
|
|
|---|
| BDBM50121040 |
|---|
| n/a |
|---|
| Name | BDBM50121040 |
|---|
| Synonyms: | CHEMBL117928 | [(S)-2-Pyridin-3-yl-1-((5S,6R)-4,4,7-trioxo-4lambda*6*-thia-1-aza-bicyclo[3.2.0]hept-6-ylcarbamoyl)-ethyl]-carbamic acid benzyl ester |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C21H22N4O6S |
|---|
| Mol. Mass. | 458.488 |
|---|
| SMILES | O=C(N[C@@H](Cc1cccnc1)C(=O)N[C@H]1[C@H]2N(CCS2(=O)=O)C1=O)OCc1ccccc1 |
|---|
| Structure | 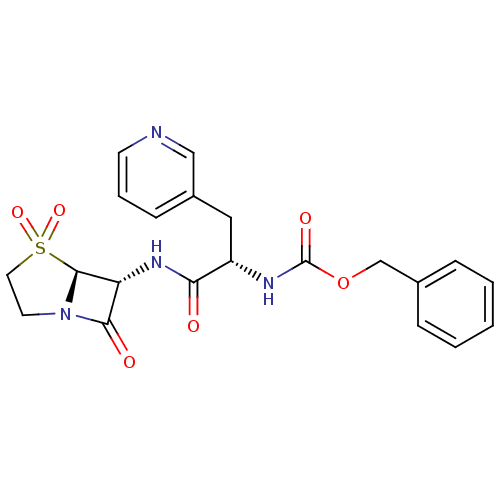
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Zhou, NE; Kaleta, J; Purisima, E; Menard, R; Micetich, RG; Singh, R 6-Acylamino-penam derivatives: synthesis and inhibition of cathepsins B, L, K, and S. Bioorg Med Chem Lett12:3417-9 (2002) [PubMed]
Zhou, NE; Kaleta, J; Purisima, E; Menard, R; Micetich, RG; Singh, R 6-Acylamino-penam derivatives: synthesis and inhibition of cathepsins B, L, K, and S. Bioorg Med Chem Lett12:3417-9 (2002) [PubMed]