| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Coagulation factor VII |
|---|
| Ligand | BDBM50126571 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEBML_48455 |
|---|
| Ki | 27±n/a nM |
|---|
| Citation |  Klingler, O; Matter, H; Schudok, M; Bajaj, SP; Czech, J; Lorenz, M; Nestler, HP; Schreuder, H; Wildgoose, P Design, synthesis, and structure-activity relationship of a new class of amidinophenylurea-based factor VIIa inhibitors. Bioorg Med Chem Lett13:1463-7 (2003) [PubMed] Klingler, O; Matter, H; Schudok, M; Bajaj, SP; Czech, J; Lorenz, M; Nestler, HP; Schreuder, H; Wildgoose, P Design, synthesis, and structure-activity relationship of a new class of amidinophenylurea-based factor VIIa inhibitors. Bioorg Med Chem Lett13:1463-7 (2003) [PubMed] |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Coagulation factor VII |
|---|
| Name: | Coagulation factor VII |
|---|
| Synonyms: | Eptacog alfa | F7 | FA7_HUMAN | Factor VIIa | Factor VIIa (fVIIa) | Proconvertin | SPCA | Thrombin and coagulation factor VII | serum prothrombin conversion accelerator |
|---|
| Type: | Enzyme |
|---|
| Mol. Mass.: | 51599.89 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | n/a |
|---|
| Residue: | 466 |
|---|
| Sequence: | MVSQALRLLCLLLGLQGCLAAGGVAKASGGETRDMPWKPGPHRVFVTQEEAHGVLHRRRR
ANAFLEELRPGSLERECKEEQCSFEEAREIFKDAERTKLFWISYSDGDQCASSPCQNGGS
CKDQLQSYICFCLPAFEGRNCETHKDDQLICVNENGGCEQYCSDHTGTKRSCRCHEGYSL
LADGVSCTPTVEYPCGKIPILEKRNASKPQGRIVGGKVCPKGECPWQVLLLVNGAQLCGG
TLINTIWVVSAAHCFDKIKNWRNLIAVLGEHDLSEHDGDEQSRRVAQVIIPSTYVPGTTN
HDIALLRLHQPVVLTDHVVPLCLPERTFSERTLAFVRFSLVSGWGQLLDRGATALELMVL
NVPRLMTQDCLQQSRKVGDSPNITEYMFCAGYSDGSKDSCKGDSGGPHATHYRGTWYLTG
IVSWGQGCATVGHFGVYTRVSQYIEWLQKLMRSEPRPGVLLRAPFP
|
|
|
|---|
| BDBM50126571 |
|---|
| n/a |
|---|
| Name | BDBM50126571 |
|---|
| Synonyms: | CHEMBL35586 | N-[(S)-1-(3-Bromo-phenyl)-ethyl]-2-[3-(4-carbamimidoyl-phenyl)-ureido]-acetamide | N-[1-((S)-3-Bromo-phenyl)-ethyl]-2-[3-(4-carbamimidoyl-phenyl)-ureido]-acetamide |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C18H20BrN5O2 |
|---|
| Mol. Mass. | 418.288 |
|---|
| SMILES | C[C@H](NC(=O)CNC(=O)Nc1ccc(cc1)C(N)=N)c1cccc(Br)c1 |
|---|
| Structure | 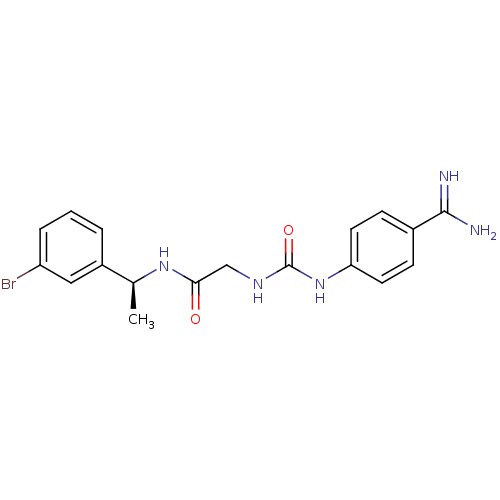
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Klingler, O; Matter, H; Schudok, M; Bajaj, SP; Czech, J; Lorenz, M; Nestler, HP; Schreuder, H; Wildgoose, P Design, synthesis, and structure-activity relationship of a new class of amidinophenylurea-based factor VIIa inhibitors. Bioorg Med Chem Lett13:1463-7 (2003) [PubMed]
Klingler, O; Matter, H; Schudok, M; Bajaj, SP; Czech, J; Lorenz, M; Nestler, HP; Schreuder, H; Wildgoose, P Design, synthesis, and structure-activity relationship of a new class of amidinophenylurea-based factor VIIa inhibitors. Bioorg Med Chem Lett13:1463-7 (2003) [PubMed]