| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Tyrosine-protein kinase Lck |
|---|
| Ligand | BDBM13357 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_221493 (CHEMBL841380) |
|---|
| Ki | 0.900000±n/a nM |
|---|
| Citation |  Das, J; Moquin, RV; Lin, J; Liu, C; Doweyko, AM; DeFex, HF; Fang, Q; Pang, S; Pitt, S; Shen, DR; Schieven, GL; Barrish, JC; Wityak, J Discovery of 2-amino-heteroaryl-benzothiazole-6-anilides as potent p56(lck) inhibitors. Bioorg Med Chem Lett13:2587-90 (2003) [PubMed] Das, J; Moquin, RV; Lin, J; Liu, C; Doweyko, AM; DeFex, HF; Fang, Q; Pang, S; Pitt, S; Shen, DR; Schieven, GL; Barrish, JC; Wityak, J Discovery of 2-amino-heteroaryl-benzothiazole-6-anilides as potent p56(lck) inhibitors. Bioorg Med Chem Lett13:2587-90 (2003) [PubMed] |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Tyrosine-protein kinase Lck |
|---|
| Name: | Tyrosine-protein kinase Lck |
|---|
| Synonyms: | 2.7.10.2 | LCK | LCK_HUMAN | LSK | Leukocyte C-terminal Src kinase | Lymphocyte cell-specific protein-tyrosine kinase | Lymphocyte-specific protein tyrosine kinase | P56-LCK | Protein YT16 | Proto-oncogene Lck | Proto-oncogene tyrosine-protein kinase LCK | Src/Lck kinase | T cell-specific protein-tyrosine kinase |
|---|
| Type: | n/a |
|---|
| Mol. Mass.: | 57987.83 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | P06239 |
|---|
| Residue: | 509 |
|---|
| Sequence: | MGCGCSSHPEDDWMENIDVCENCHYPIVPLDGKGTLLIRNGSEVRDPLVTYEGSNPPASP
LQDNLVIALHSYEPSHDGDLGFEKGEQLRILEQSGEWWKAQSLTTGQEGFIPFNFVAKAN
SLEPEPWFFKNLSRKDAERQLLAPGNTHGSFLIRESESTAGSFSLSVRDFDQNQGEVVKH
YKIRNLDNGGFYISPRITFPGLHELVRHYTNASDGLCTRLSRPCQTQKPQKPWWEDEWEV
PRETLKLVERLGAGQFGEVWMGYYNGHTKVAVKSLKQGSMSPDAFLAEANLMKQLQHQRL
VRLYAVVTQEPIYIITEYMENGSLVDFLKTPSGIKLTINKLLDMAAQIAEGMAFIEERNY
IHRDLRAANILVSDTLSCKIADFGLARLIEDNEYTAREGAKFPIKWTAPEAINYGTFTIK
SDVWSFGILLTEIVTHGRIPYPGMTNPEVIQNLERGYRMVRPDNCPEELYQLMRLCWKER
PEDRPTFDYLRSVLEDFFTATEGQYQPQP
|
|
|
|---|
| BDBM13357 |
|---|
| n/a |
|---|
| Name | BDBM13357 |
|---|
| Synonyms: | CHEMBL312933 | N-(2-chloro-6-methylphenyl)-2-({6-[(2-hydroxyethyl)amino]pyridin-2-yl}amino)-1,3-benzothiazole-6-carboxamide | benzothiazole analog 13a |
|---|
| Type | Small Organic Molecule |
|---|
| Emp. Form. | C22H20ClN5O2S |
|---|
| Mol. Mass. | 453.945 |
|---|
| SMILES | Cc1cccc(Cl)c1NC(=O)c1ccc2nc(Nc3cccc(NCCO)n3)sc2c1 |
|---|
| Structure | 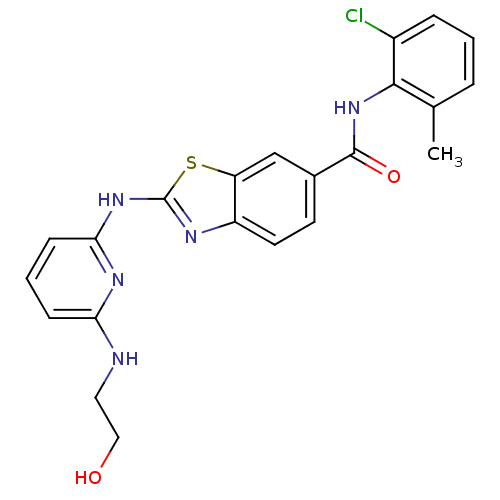
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Das, J; Moquin, RV; Lin, J; Liu, C; Doweyko, AM; DeFex, HF; Fang, Q; Pang, S; Pitt, S; Shen, DR; Schieven, GL; Barrish, JC; Wityak, J Discovery of 2-amino-heteroaryl-benzothiazole-6-anilides as potent p56(lck) inhibitors. Bioorg Med Chem Lett13:2587-90 (2003) [PubMed]
Das, J; Moquin, RV; Lin, J; Liu, C; Doweyko, AM; DeFex, HF; Fang, Q; Pang, S; Pitt, S; Shen, DR; Schieven, GL; Barrish, JC; Wityak, J Discovery of 2-amino-heteroaryl-benzothiazole-6-anilides as potent p56(lck) inhibitors. Bioorg Med Chem Lett13:2587-90 (2003) [PubMed]