| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Genome polyprotein |
|---|
| Ligand | BDBM50131396 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEBML_143492 |
|---|
| IC50 | 54±n/a nM |
|---|
| Citation |  Orvieto, F; Koch, U; Matassa, VG; Muraglia, E Novel, potent phenethylamide inhibitors of the hepatitis C virus (HCV) NS3 protease: probing the role of P2 aryloxyprolines with hybrid structures. Bioorg Med Chem Lett13:2745-8 (2003) [PubMed] Orvieto, F; Koch, U; Matassa, VG; Muraglia, E Novel, potent phenethylamide inhibitors of the hepatitis C virus (HCV) NS3 protease: probing the role of P2 aryloxyprolines with hybrid structures. Bioorg Med Chem Lett13:2745-8 (2003) [PubMed] |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Genome polyprotein |
|---|
| Name: | Genome polyprotein |
|---|
| Synonyms: | Hepatitis C virus NS3 protease/helicase | Hepatitis C virus serine protease, NS3/NS4A |
|---|
| Type: | Protein |
|---|
| Mol. Mass.: | 67067.41 |
|---|
| Organism: | Hepatitis C virus |
|---|
| Description: | A3EZI9 |
|---|
| Residue: | 631 |
|---|
| Sequence: | APITAYAQQTRGLLGCIITSLTGRDKNQVEGEVQIVSTAAQTFLATCINGVCWTVYHGAG
TRTIASSKGPVIQMYTNVDQDLVGWPAPQGARSLTPCTCGSSDLYLVTRHADVIPVRRRG
DGRGSLLSPRPISYLKGSSGGPLLCPAGHAVGIFRAAVCTRGVAKAVDFIPVEGLETTMR
SPVFSDNSSPPAVPQSYQVAHLHAPTGSGKSTKVPAAYAAQGYKVLVLNPSVAATLGFGA
YMSKAHGIDPNIRTGVRTITTGSPITYSTYGKFLADGGCSGGAYDIIICDECHSTDATSI
LGIGTVLDQAETAGARLTVLATATPPGSVTVPHPNIEEVALSTTGEIPFYGKAIPLEAIK
GGRHLIFCHSKKKCDELAAKLVALGVNAVAYYRGLDVSVIPASGDVVVVATDALMTGFTG
DFDSVIDCNTCVTQTVDFSLDPTFTIETTTLPQDAVSRTQRRGRTGRGKPGIYRFVTPGE
RPSGMFDSSVLCECYDAGCAWYELTPAETTVRLRAYMNTPGLPVCQDHLEFWEGVFTGLT
HIDAHFLSQTKQSGENLPYLVAYQATVCARAQAPPPSWDQMWKCLIRLKPTLHGPTPLLY
RLGAVQNEITLTHPITKYIMTCMSADLEVVT
|
|
|
|---|
| BDBM50131396 |
|---|
| n/a |
|---|
| Name | BDBM50131396 |
|---|
| Synonyms: | 4-[2-(2-{[(2S,4R)-1-((S)-2-tert-Butoxycarbonylamino-3,3-dimethyl-butyryl)-4-(7-methoxy-2-phenyl-quinolin-4-yloxy)-pyrrolidine-2-carbonyl]-amino}-4,4-difluoro-butyrylamino)-ethyl]-3,5-difluoro-benzoic acid | CHEMBL317487 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C45H51F4N5O9 |
|---|
| Mol. Mass. | 881.9082 |
|---|
| SMILES | COc1ccc2c(O[C@@H]3C[C@H](N(C3)C(=O)[C@@H](NC(=O)OC(C)(C)C)C(C)(C)C)C(=O)NC(CC(F)F)C(=O)NCCc3c(F)cc(cc3F)C(O)=O)cc(nc2c1)-c1ccccc1 |
|---|
| Structure | 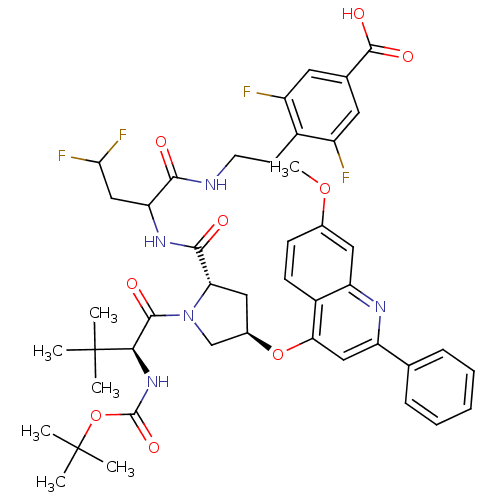
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Orvieto, F; Koch, U; Matassa, VG; Muraglia, E Novel, potent phenethylamide inhibitors of the hepatitis C virus (HCV) NS3 protease: probing the role of P2 aryloxyprolines with hybrid structures. Bioorg Med Chem Lett13:2745-8 (2003) [PubMed]
Orvieto, F; Koch, U; Matassa, VG; Muraglia, E Novel, potent phenethylamide inhibitors of the hepatitis C virus (HCV) NS3 protease: probing the role of P2 aryloxyprolines with hybrid structures. Bioorg Med Chem Lett13:2745-8 (2003) [PubMed]