| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Melanocortin receptor 5 |
|---|
| Ligand | BDBM50134951 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_106655 (CHEMBL713000) |
|---|
| Ki | 8.8±n/a nM |
|---|
| Citation |  Balse-Srinivasan, P; Grieco, P; Cai, M; Trivedi, D; Hruby, VJ Structure-activity relationships of gamma-MSH analogues at the human melanocortin MC3, MC4, and MC5 receptors. Discovery of highly selective hMC3R, hMC4R, and hMC5R analogues. J Med Chem46:4965-73 (2003) [PubMed] Article Balse-Srinivasan, P; Grieco, P; Cai, M; Trivedi, D; Hruby, VJ Structure-activity relationships of gamma-MSH analogues at the human melanocortin MC3, MC4, and MC5 receptors. Discovery of highly selective hMC3R, hMC4R, and hMC5R analogues. J Med Chem46:4965-73 (2003) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Melanocortin receptor 5 |
|---|
| Name: | Melanocortin receptor 5 |
|---|
| Synonyms: | MC-2 | MC5-R | MC5R | MC5R_HUMAN | Melanocortin MC5 | Melanocortin receptor (M4 and M5) | Melanocortin receptor 5 | Melanocortin receptor 5 (MC5R) |
|---|
| Type: | Enzyme |
|---|
| Mol. Mass.: | 36612.92 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | P33032 |
|---|
| Residue: | 325 |
|---|
| Sequence: | MNSSFHLHFLDLNLNATEGNLSGPNVKNKSSPCEDMGIAVEVFLTLGVISLLENILVIGA
IVKNKNLHSPMYFFVCSLAVADMLVSMSSAWETITIYLLNNKHLVIADAFVRHIDNVFDS
MICISVVASMCSLLAIAVDRYVTIFYALRYHHIMTARRSGAIIAGIWAFCTGCGIVFILY
SESTYVILCLISMFFAMLFLLVSLYIHMFLLARTHVKRIAALPGASSARQRTSMQGAVTV
TMLLGVFTVCWAPFFLHLTLMLSCPQNLYCSRFMSHFNMYLILIMCNSVMDPLIYAFRSQ
EMRKTFKEIICCRGFRIACSFPRRD
|
|
|
|---|
| BDBM50134951 |
|---|
| n/a |
|---|
| Name | BDBM50134951 |
|---|
| Synonyms: | CHEMBL409636 | H-Tyr-Val-Nle-c[Asp-His-Phe-Arg-Trp-Lys]-Arg-Phe-Gly-NH2 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C79H109N23O14 |
|---|
| Mol. Mass. | 1604.8565 |
|---|
| SMILES | CCCC[C@H](NC(=O)[C@@H](NC(=O)[C@@H](N)Cc1ccc(O)cc1)C(C)C)C(=O)N[C@H]1CC(=O)NCCCC[C@@H](NC(=O)[C@@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H](Cc2ccccc2)NC(=O)[C@H](Cc2cnc[nH]2)NC1=O)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](Cc1ccccc1)C(=O)NCC(N)=O |
|---|
| Structure | 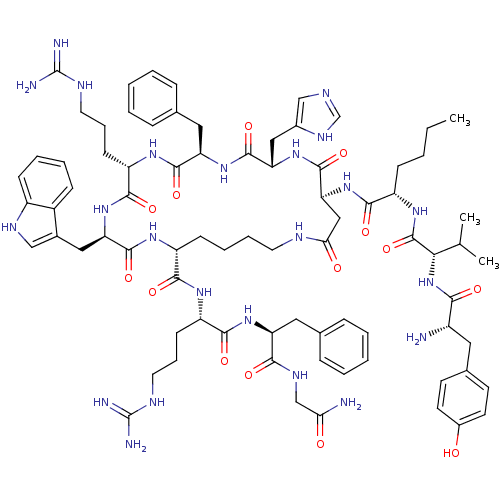
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Balse-Srinivasan, P; Grieco, P; Cai, M; Trivedi, D; Hruby, VJ Structure-activity relationships of gamma-MSH analogues at the human melanocortin MC3, MC4, and MC5 receptors. Discovery of highly selective hMC3R, hMC4R, and hMC5R analogues. J Med Chem46:4965-73 (2003) [PubMed] Article
Balse-Srinivasan, P; Grieco, P; Cai, M; Trivedi, D; Hruby, VJ Structure-activity relationships of gamma-MSH analogues at the human melanocortin MC3, MC4, and MC5 receptors. Discovery of highly selective hMC3R, hMC4R, and hMC5R analogues. J Med Chem46:4965-73 (2003) [PubMed] Article