| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Carboxypeptidase B2 |
|---|
| Ligand | BDBM50135934 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_45639 (CHEMBL655449) |
|---|
| IC50 | 2.7±n/a nM |
|---|
| Citation |  Barrow, JC; Nantermet, PG; Stauffer, SR; Ngo, PL; Steinbeiser, MA; Mao, SS; Carroll, SS; Bailey, C; Colussi, D; Bosserman, M; Burlein, C; Cook, JJ; Sitko, G; Tiller, PR; Miller-Stein, CM; Rose, M; McMasters, DR; Vacca, JP; Selnick, HG Synthesis and evaluation of imidazole acetic acid inhibitors of activated thrombin-activatable fibrinolysis inhibitor as novel antithrombotics. J Med Chem46:5294-7 (2003) [PubMed] Article Barrow, JC; Nantermet, PG; Stauffer, SR; Ngo, PL; Steinbeiser, MA; Mao, SS; Carroll, SS; Bailey, C; Colussi, D; Bosserman, M; Burlein, C; Cook, JJ; Sitko, G; Tiller, PR; Miller-Stein, CM; Rose, M; McMasters, DR; Vacca, JP; Selnick, HG Synthesis and evaluation of imidazole acetic acid inhibitors of activated thrombin-activatable fibrinolysis inhibitor as novel antithrombotics. J Med Chem46:5294-7 (2003) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Carboxypeptidase B2 |
|---|
| Name: | Carboxypeptidase B2 |
|---|
| Synonyms: | CBPB2_HUMAN | CPB2 | CPU | Carboxypeptidase B2 | Carboxypeptidase B2 isoform A | Carboxypeptidase U | Plasma carboxypeptidase B | TAFI | Thrombin-activable fibrinolysis inhibitor | pCPB |
|---|
| Type: | Enzyme |
|---|
| Mol. Mass.: | 48432.74 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | Q96IY4 |
|---|
| Residue: | 423 |
|---|
| Sequence: | MKLCSLAVLVPIVLFCEQHVFAFQSGQVLAALPRTSRQVQVLQNLTTTYEIVLWQPVTAD
LIVKKKQVHFFVNASDVDNVKAHLNVSGIPCSVLLADVEDLIQQQISNDTVSPRASASYY
EQYHSLNEIYSWIEFITERHPDMLTKIHIGSSFEKYPLYVLKVSGKEQAAKNAIWIDCGI
HAREWISPAFCLWFIGHITQFYGIIGQYTNLLRLVDFYVMPVVNVDGYDYSWKKNRMWRK
NRSFYANNHCIGTDLNRNFASKHWCEEGASSSSCSETYCGLYPESEPEVKAVASFLRRNI
NQIKAYISMHSYSQHIVFPYSYTRSKSKDHEELSLVASEAVRAIEKISKNTRYTHGHGSE
TLYLAPGGGDDWIYDLGIKYSFTIELRDTGTYGFLLPERYIKPTCREAFAAVSKIAWHVI
RNV
|
|
|
|---|
| BDBM50135934 |
|---|
| n/a |
|---|
| Name | BDBM50135934 |
|---|
| Synonyms: | 3-(6-Amino-pyridin-3-yl)-2-[1-(3-methyl-butyl)-1H-imidazol-4-yl]-propionic acid | CHEMBL68399 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C16H22N4O2 |
|---|
| Mol. Mass. | 302.3715 |
|---|
| SMILES | CC(C)CCn1cnc(c1)C(Cc1ccc(N)nc1)C(O)=O |
|---|
| Structure | 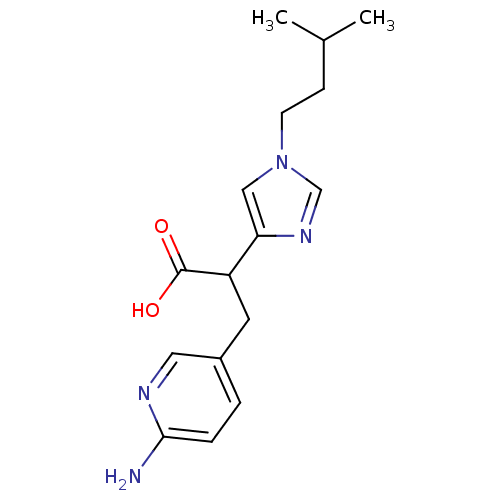
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Barrow, JC; Nantermet, PG; Stauffer, SR; Ngo, PL; Steinbeiser, MA; Mao, SS; Carroll, SS; Bailey, C; Colussi, D; Bosserman, M; Burlein, C; Cook, JJ; Sitko, G; Tiller, PR; Miller-Stein, CM; Rose, M; McMasters, DR; Vacca, JP; Selnick, HG Synthesis and evaluation of imidazole acetic acid inhibitors of activated thrombin-activatable fibrinolysis inhibitor as novel antithrombotics. J Med Chem46:5294-7 (2003) [PubMed] Article
Barrow, JC; Nantermet, PG; Stauffer, SR; Ngo, PL; Steinbeiser, MA; Mao, SS; Carroll, SS; Bailey, C; Colussi, D; Bosserman, M; Burlein, C; Cook, JJ; Sitko, G; Tiller, PR; Miller-Stein, CM; Rose, M; McMasters, DR; Vacca, JP; Selnick, HG Synthesis and evaluation of imidazole acetic acid inhibitors of activated thrombin-activatable fibrinolysis inhibitor as novel antithrombotics. J Med Chem46:5294-7 (2003) [PubMed] Article