| Reaction Details |
|---|
 | Report a problem with these data |
| Target | 5-hydroxytryptamine receptor 1D |
|---|
| Ligand | BDBM50136471 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEBML_1724 |
|---|
| Ki | 4.6±n/a nM |
|---|
| Citation |  Isaac, M; Slassi, M; Xin, T; Arora, J; O'Brien, A; Edwards, L; MacLean, N; Wilson, J; Demschyshyn, L; Labrie, P; Naismith, A; Maddaford, S; Papac, D; Harrison, S; Wang, H; Draper, S; Tehim, A Design, synthesis and biological activity of novel dimethyl-[2-[6-substituted-indol-1-yl]-ethyl]-amine as potent, selective, and orally-bioavailable 5-HT(1D) agonists. Bioorg Med Chem Lett13:4409-13 (2003) [PubMed] Isaac, M; Slassi, M; Xin, T; Arora, J; O'Brien, A; Edwards, L; MacLean, N; Wilson, J; Demschyshyn, L; Labrie, P; Naismith, A; Maddaford, S; Papac, D; Harrison, S; Wang, H; Draper, S; Tehim, A Design, synthesis and biological activity of novel dimethyl-[2-[6-substituted-indol-1-yl]-ethyl]-amine as potent, selective, and orally-bioavailable 5-HT(1D) agonists. Bioorg Med Chem Lett13:4409-13 (2003) [PubMed] |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| 5-hydroxytryptamine receptor 1D |
|---|
| Name: | 5-hydroxytryptamine receptor 1D |
|---|
| Synonyms: | 5-HT-1D | 5-HT-1D-alpha | 5-HT1D | 5-hydroxytryptamine receptor 1D (5-HT1D) | 5HT1D_HUMAN | HTR1D | HTR1DA | HTRL | Serotonin (5-HT) receptor | Serotonin Receptor 1D |
|---|
| Type: | G Protein-Coupled Receptor (GPCR) |
|---|
| Mol. Mass.: | 41920.63 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | Receptor binding assays were performed using human clone stably expressed in CHO cells. |
|---|
| Residue: | 377 |
|---|
| Sequence: | MSPLNQSAEGLPQEASNRSLNATETSEAWDPRTLQALKISLAVVLSVITLATVLSNAFVL
TTILLTRKLHTPANYLIGSLATTDLLVSILVMPISIAYTITHTWNFGQILCDIWLSSDIT
CCTASILHLCVIALDRYWAITDALEYSKRRTAGHAATMIAIVWAISICISIPPLFWRQAK
AQEEMSDCLVNTSQISYTIYSTCGAFYIPSVLLIILYGRIYRAARNRILNPPSLYGKRFT
TAHLITGSAGSSLCSLNSSLHEGHSHSAGSPLFFNHVKIKLADSALERKRISAARERKAT
KILGIILGAFIICWLPFFVVSLVLPICRDSCWIHPALFDFFTWLGYLNSLINPIIYTVFN
EEFRQAFQKIVPFRKAS
|
|
|
|---|
| BDBM50136471 |
|---|
| n/a |
|---|
| Name | BDBM50136471 |
|---|
| Synonyms: | (S)-1-{2-[4-(4-Carbamoyl-phenyl)-piperazin-1-yl]-ethyl}-isochroman-6-carboxylic acid methylamide | CHEMBL441095 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C24H30N4O3 |
|---|
| Mol. Mass. | 422.52 |
|---|
| SMILES | CNC(=O)c1ccc2[C@H](CCN3CCN(CC3)c3ccc(cc3)C(N)=O)OCCc2c1 |
|---|
| Structure | 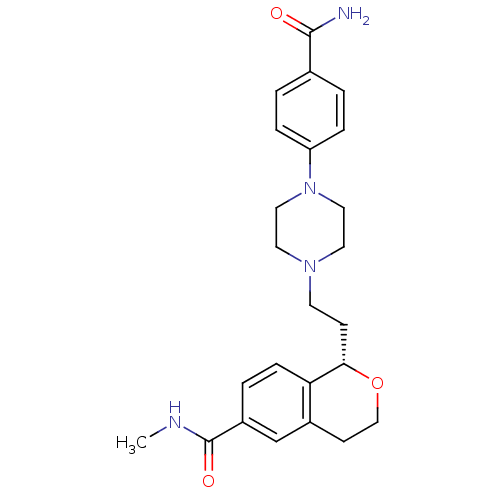
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Isaac, M; Slassi, M; Xin, T; Arora, J; O'Brien, A; Edwards, L; MacLean, N; Wilson, J; Demschyshyn, L; Labrie, P; Naismith, A; Maddaford, S; Papac, D; Harrison, S; Wang, H; Draper, S; Tehim, A Design, synthesis and biological activity of novel dimethyl-[2-[6-substituted-indol-1-yl]-ethyl]-amine as potent, selective, and orally-bioavailable 5-HT(1D) agonists. Bioorg Med Chem Lett13:4409-13 (2003) [PubMed]
Isaac, M; Slassi, M; Xin, T; Arora, J; O'Brien, A; Edwards, L; MacLean, N; Wilson, J; Demschyshyn, L; Labrie, P; Naismith, A; Maddaford, S; Papac, D; Harrison, S; Wang, H; Draper, S; Tehim, A Design, synthesis and biological activity of novel dimethyl-[2-[6-substituted-indol-1-yl]-ethyl]-amine as potent, selective, and orally-bioavailable 5-HT(1D) agonists. Bioorg Med Chem Lett13:4409-13 (2003) [PubMed]