| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Cytochrome P450 1A2 |
|---|
| Ligand | BDBM50138599 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEBML_51352 |
|---|
| IC50 | >100000±n/a nM |
|---|
| Citation |  Koltun, DO; Marquart, TA; Shenk, KD; Elzein, E; Li, Y; Nguyen, M; Kerwar, S; Zeng, D; Chu, N; Soohoo, D; Hao, J; Maydanik, VY; Lustig, DA; Ng, KJ; Fraser, H; Zablocki, JA New fatty acid oxidation inhibitors with increased potency lacking adverse metabolic and electrophysiological properties. Bioorg Med Chem Lett14:549-52 (2003) [PubMed] Koltun, DO; Marquart, TA; Shenk, KD; Elzein, E; Li, Y; Nguyen, M; Kerwar, S; Zeng, D; Chu, N; Soohoo, D; Hao, J; Maydanik, VY; Lustig, DA; Ng, KJ; Fraser, H; Zablocki, JA New fatty acid oxidation inhibitors with increased potency lacking adverse metabolic and electrophysiological properties. Bioorg Med Chem Lett14:549-52 (2003) [PubMed] |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Cytochrome P450 1A2 |
|---|
| Name: | Cytochrome P450 1A2 |
|---|
| Synonyms: | CP1A2_HUMAN | CYP1A2 | CYPIA2 | Cholesterol 25-hydroxylase | Cytochrome P(3)450 | Cytochrome P450 1A | Cytochrome P450 1A2 (CYP1A2) | Cytochrome P450 4 | Cytochrome P450-P3 |
|---|
| Type: | Enzyme |
|---|
| Mol. Mass.: | 58423.38 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | P05177 |
|---|
| Residue: | 516 |
|---|
| Sequence: | MALSQSVPFSATELLLASAIFCLVFWVLKGLRPRVPKGLKSPPEPWGWPLLGHVLTLGKN
PHLALSRMSQRYGDVLQIRIGSTPVLVLSRLDTIRQALVRQGDDFKGRPDLYTSTLITDG
QSLTFSTDSGPVWAARRRLAQNALNTFSIASDPASSSSCYLEEHVSKEAKALISRLQELM
AGPGHFDPYNQVVVSVANVIGAMCFGQHFPESSDEMLSLVKNTHEFVETASSGNPLDFFP
ILRYLPNPALQRFKAFNQRFLWFLQKTVQEHYQDFDKNSVRDITGALFKHSKKGPRASGN
LIPQEKIVNLVNDIFGAGFDTVTTAISWSLMYLVTKPEIQRKIQKELDTVIGRERRPRLS
DRPQLPYLEAFILETFRHSSFLPFTIPHSTTRDTTLNGFYIPKKCCVFVNQWQVNHDPEL
WEDPSEFRPERFLTADGTAINKPLSEKMMLFGMGKRRCIGEVLAKWEIFLFLAILLQQLE
FSVPPGVKVDLTPIYGLTMKHARCEHVQARLRFSIN
|
|
|
|---|
| BDBM50138599 |
|---|
| n/a |
|---|
| Name | BDBM50138599 |
|---|
| Synonyms: | CHEMBL154751 | N-(2,6-Dimethyl-phenyl)-2-{4-[(R)-2-hydroxy-3-(2-methyl-benzothiazol-5-yloxy)-propyl]-piperazin-1-yl}-acetamide |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C25H32N4O3S |
|---|
| Mol. Mass. | 468.612 |
|---|
| SMILES | Cc1nc2cc(OC[C@H](O)CN3CCN(CC(=O)Nc4c(C)cccc4C)CC3)ccc2s1 |
|---|
| Structure | 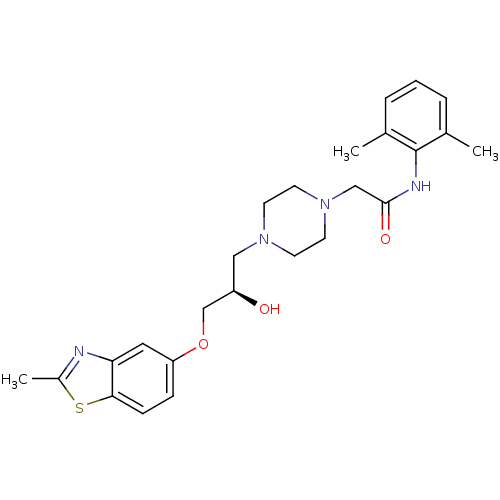
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Koltun, DO; Marquart, TA; Shenk, KD; Elzein, E; Li, Y; Nguyen, M; Kerwar, S; Zeng, D; Chu, N; Soohoo, D; Hao, J; Maydanik, VY; Lustig, DA; Ng, KJ; Fraser, H; Zablocki, JA New fatty acid oxidation inhibitors with increased potency lacking adverse metabolic and electrophysiological properties. Bioorg Med Chem Lett14:549-52 (2003) [PubMed]
Koltun, DO; Marquart, TA; Shenk, KD; Elzein, E; Li, Y; Nguyen, M; Kerwar, S; Zeng, D; Chu, N; Soohoo, D; Hao, J; Maydanik, VY; Lustig, DA; Ng, KJ; Fraser, H; Zablocki, JA New fatty acid oxidation inhibitors with increased potency lacking adverse metabolic and electrophysiological properties. Bioorg Med Chem Lett14:549-52 (2003) [PubMed]