| Reaction Details |
|---|
 | Report a problem with these data |
| Target | 1-acyl-sn-glycerol-3-phosphate acyltransferase beta |
|---|
| Ligand | BDBM50141255 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEBML_100169 |
|---|
| IC50 | 15±n/a nM |
|---|
| Citation |  Gong, B; Hong, F; Kohm, C; Bonham, L; Klein, P Synthesis and SAR of 2-arylbenzoxazoles, benzothiazoles and benzimidazoles as inhibitors of lysophosphatidic acid acyltransferase-beta. Bioorg Med Chem Lett14:1455-9 (2004) [PubMed] Article Gong, B; Hong, F; Kohm, C; Bonham, L; Klein, P Synthesis and SAR of 2-arylbenzoxazoles, benzothiazoles and benzimidazoles as inhibitors of lysophosphatidic acid acyltransferase-beta. Bioorg Med Chem Lett14:1455-9 (2004) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| 1-acyl-sn-glycerol-3-phosphate acyltransferase beta |
|---|
| Name: | 1-acyl-sn-glycerol-3-phosphate acyltransferase beta |
|---|
| Synonyms: | 1-acylglycerol-3-phosphate O-acyltransferase beta | AGPAT2 | PLCB_HUMAN |
|---|
| Type: | PROTEIN |
|---|
| Mol. Mass.: | 30924.09 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | ChEMBL_321473 |
|---|
| Residue: | 278 |
|---|
| Sequence: | MELWPCLAAALLLLLLLVQLSRAAEFYAKVALYCALCFTVSAVASLVCLLRHGGRTVENM
SIIGWFVRSFKYFYGLRFEVRDPRRLQEARPCVIVSNHQSILDMMGLMEVLPERCVQIAK
RELLFLGPVGLIMYLGGVFFINRQRSSTAMTVMADLGERMVRENLKVWIYPEGTRNDNGD
LLPFKKGAFYLAVQAQVPIVPVVYSSFSSFYNTKKKFFTSGTVTVQVLEAIPTSGLTAAD
VPALVDTCHRAMRTTFLHISKTPQENGATAGSGVQPAQ
|
|
|
|---|
| BDBM50141255 |
|---|
| n/a |
|---|
| Name | BDBM50141255 |
|---|
| Synonyms: | CHEMBL35508 | [3-(5-Chloro-benzothiazol-2-yl)-4-methyl-phenyl]-carbamic acid prop-2-ynyl ester |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C18H13ClN2O2S |
|---|
| Mol. Mass. | 356.826 |
|---|
| SMILES | Cc1ccc(NC(=O)OCC#C)cc1-c1nc2cc(Cl)ccc2s1 |
|---|
| Structure | 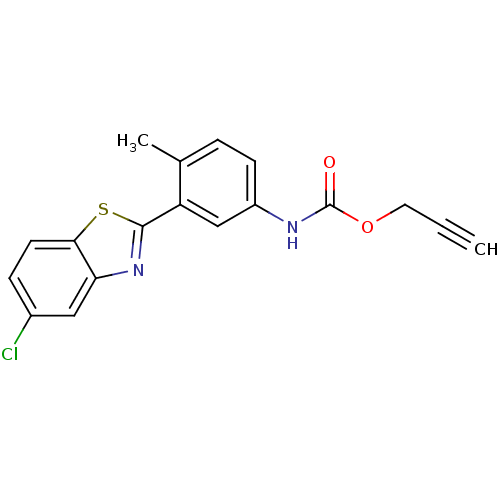
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Gong, B; Hong, F; Kohm, C; Bonham, L; Klein, P Synthesis and SAR of 2-arylbenzoxazoles, benzothiazoles and benzimidazoles as inhibitors of lysophosphatidic acid acyltransferase-beta. Bioorg Med Chem Lett14:1455-9 (2004) [PubMed] Article
Gong, B; Hong, F; Kohm, C; Bonham, L; Klein, P Synthesis and SAR of 2-arylbenzoxazoles, benzothiazoles and benzimidazoles as inhibitors of lysophosphatidic acid acyltransferase-beta. Bioorg Med Chem Lett14:1455-9 (2004) [PubMed] Article