| Reaction Details |
|---|
 | Report a problem with these data |
| Target | C-C chemokine receptor type 5 |
|---|
| Ligand | BDBM50145682 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_39636 (CHEMBL649943) |
|---|
| Ki | 5.2±n/a nM |
|---|
| Citation |  Tagat, JR; McCombie, SW; Nazareno, D; Labroli, MA; Xiao, Y; Steensma, RW; Strizki, JM; Baroudy, BM; Cox, K; Lachowicz, J; Varty, G; Watkins, R Piperazine-based CCR5 antagonists as HIV-1 inhibitors. IV. Discovery of 1-[(4,6-dimethyl-5-pyrimidinyl)carbonyl]- 4-[4-[2-methoxy-1(R)-4-(trifluoromethyl)phenyl]ethyl-3(S)-methyl-1-piperazinyl]- 4-methylpiperidine (Sch-417690/Sch-D), a potent, highly selective, and orally bioavailable CCR5 antagoni J Med Chem47:2405-8 (2004) [PubMed] Article Tagat, JR; McCombie, SW; Nazareno, D; Labroli, MA; Xiao, Y; Steensma, RW; Strizki, JM; Baroudy, BM; Cox, K; Lachowicz, J; Varty, G; Watkins, R Piperazine-based CCR5 antagonists as HIV-1 inhibitors. IV. Discovery of 1-[(4,6-dimethyl-5-pyrimidinyl)carbonyl]- 4-[4-[2-methoxy-1(R)-4-(trifluoromethyl)phenyl]ethyl-3(S)-methyl-1-piperazinyl]- 4-methylpiperidine (Sch-417690/Sch-D), a potent, highly selective, and orally bioavailable CCR5 antagoni J Med Chem47:2405-8 (2004) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| C-C chemokine receptor type 5 |
|---|
| Name: | C-C chemokine receptor type 5 |
|---|
| Synonyms: | C-C CKR-5 | C-C chemokine receptor type 5 | CC-CKR-5 | CCR-5 | CCR5 | CCR5/mu opioid receptor complex | CCR5_HUMAN | CD_antigen=CD195 | CHEMR13 | CMKBR5 | HIV-1 fusion coreceptor |
|---|
| Type: | Enzyme |
|---|
| Mol. Mass.: | 40540.21 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | P51681 |
|---|
| Residue: | 352 |
|---|
| Sequence: | MDYQVSSPIYDINYYTSEPCQKINVKQIAARLLPPLYSLVFIFGFVGNMLVILILINCKR
LKSMTDIYLLNLAISDLFFLLTVPFWAHYAAAQWDFGNTMCQLLTGLYFIGFFSGIFFII
LLTIDRYLAVVHAVFALKARTVTFGVVTSVITWVVAVFASLPGIIFTRSQKEGLHYTCSS
HFPYSQYQFWKNFQTLKIVILGLVLPLLVMVICYSGILKTLLRCRNEKKRHRAVRLIFTI
MIVYFLFWAPYNIVLLLNTFQEFFGLNNCSSSNRLDQAMQVTETLGMTHCCINPIIYAFV
GEKFRNYLLVFFQKHIAKRFCKCCSIFQQEAPERASSVYTRSTGEQEISVGL
|
|
|
|---|
| BDBM50145682 |
|---|
| n/a |
|---|
| Name | BDBM50145682 |
|---|
| Synonyms: | (4,6-Dimethyl-pyrimidin-5-yl)-(4-methyl-4-{(S)-3-methyl-4-[(S)-1-(4-trifluoromethyl-phenyl)-propyl]-piperazin-1-yl}-piperidin-1-yl)-methanone | CHEMBL263555 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C28H38F3N5O |
|---|
| Mol. Mass. | 517.6294 |
|---|
| SMILES | CC[C@H](N1CCN(C[C@@H]1C)C1(C)CCN(CC1)C(=O)c1c(C)ncnc1C)c1ccc(cc1)C(F)(F)F |
|---|
| Structure | 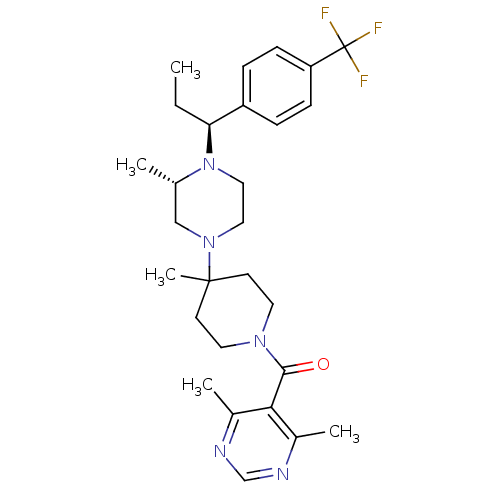
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Tagat, JR; McCombie, SW; Nazareno, D; Labroli, MA; Xiao, Y; Steensma, RW; Strizki, JM; Baroudy, BM; Cox, K; Lachowicz, J; Varty, G; Watkins, R Piperazine-based CCR5 antagonists as HIV-1 inhibitors. IV. Discovery of 1-[(4,6-dimethyl-5-pyrimidinyl)carbonyl]- 4-[4-[2-methoxy-1(R)-4-(trifluoromethyl)phenyl]ethyl-3(S)-methyl-1-piperazinyl]- 4-methylpiperidine (Sch-417690/Sch-D), a potent, highly selective, and orally bioavailable CCR5 antagoni J Med Chem47:2405-8 (2004) [PubMed] Article
Tagat, JR; McCombie, SW; Nazareno, D; Labroli, MA; Xiao, Y; Steensma, RW; Strizki, JM; Baroudy, BM; Cox, K; Lachowicz, J; Varty, G; Watkins, R Piperazine-based CCR5 antagonists as HIV-1 inhibitors. IV. Discovery of 1-[(4,6-dimethyl-5-pyrimidinyl)carbonyl]- 4-[4-[2-methoxy-1(R)-4-(trifluoromethyl)phenyl]ethyl-3(S)-methyl-1-piperazinyl]- 4-methylpiperidine (Sch-417690/Sch-D), a potent, highly selective, and orally bioavailable CCR5 antagoni J Med Chem47:2405-8 (2004) [PubMed] Article