| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Adenosine receptor A2a |
|---|
| Ligand | BDBM50151195 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_303190 (CHEMBL829684) |
|---|
| Ki | 18±n/a nM |
|---|
| Citation |  Vu, CB; Peng, B; Kumaravel, G; Smits, G; Jin, X; Phadke, D; Engber, T; Huang, C; Reilly, J; Tam, S; Grant, D; Hetu, G; Chen, L; Zhang, J; Petter, RC Piperazine derivatives of [1,2,4]triazolo[1,5-a][1,3,5]triazine as potent and selective adenosine A2a receptor antagonists. J Med Chem47:4291-9 (2004) [PubMed] Article Vu, CB; Peng, B; Kumaravel, G; Smits, G; Jin, X; Phadke, D; Engber, T; Huang, C; Reilly, J; Tam, S; Grant, D; Hetu, G; Chen, L; Zhang, J; Petter, RC Piperazine derivatives of [1,2,4]triazolo[1,5-a][1,3,5]triazine as potent and selective adenosine A2a receptor antagonists. J Med Chem47:4291-9 (2004) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Adenosine receptor A2a |
|---|
| Name: | Adenosine receptor A2a |
|---|
| Synonyms: | AA2AR_RAT | ADENOSINE A2a | Adenosine A2 receptor | Adenosine A2a receptor (A2a) | Adenosine Receptors A2a (A2a) | Adenosine receptor A2a and A3 | Adenosine receptors A2a | Adora2a | Rat striatal adenosine A2a receptor |
|---|
| Type: | G Protein-Coupled Receptor (GPCR) |
|---|
| Mol. Mass.: | 45015.65 |
|---|
| Organism: | Rattus norvegicus (rat) |
|---|
| Description: | Rat A2A receptors expressed in CHO cells. |
|---|
| Residue: | 410 |
|---|
| Sequence: | MGSSVYITVELAIAVLAILGNVLVCWAVWINSNLQNVTNFFVVSLAAADIAVGVLAIPFA
ITISTGFCAACHGCLFFACFVLVLTQSSIFSLLAIAIDRYIAIRIPLRYNGLVTGVRAKG
IIAICWVLSFAIGLTPMLGWNNCSQKDGNSTKTCGEGRVTCLFEDVVPMNYMVYYNFFAF
VLLPLLLMLAIYLRIFLAARRQLKQMESQPLPGERTRSTLQKEVHAAKSLAIIVGLFALC
WLPLHIINCFTFFCSTCRHAPPWLMYLAIILSHSNSVVNPFIYAYRIREFRQTFRKIIRT
HVLRRQEPFQAGGSSAWALAAHSTEGEQVSLRLNGHPLGVWANGSATHSGRRPNGYTLGL
GGGGSAQGSPRDVELPTQERQEGQEHPGLRGHLVQARVGASSWSSEFAPS
|
|
|
|---|
| BDBM50151195 |
|---|
| n/a |
|---|
| Name | BDBM50151195 |
|---|
| Synonyms: | 5-[4-(3,5-Dimethyl-isoxazol-4-ylmethyl)-piperazin-1-yl]-2-furan-2-yl-[1,2,4]triazolo[1,5-a][1,3,5]triazin-7-ylamine | CHEMBL368619 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C18H21N9O2 |
|---|
| Mol. Mass. | 395.4184 |
|---|
| SMILES | Cc1noc(C)c1CN1CCN(CC1)c1nc(N)n2nc(nc2n1)-c1ccco1 |
|---|
| Structure | 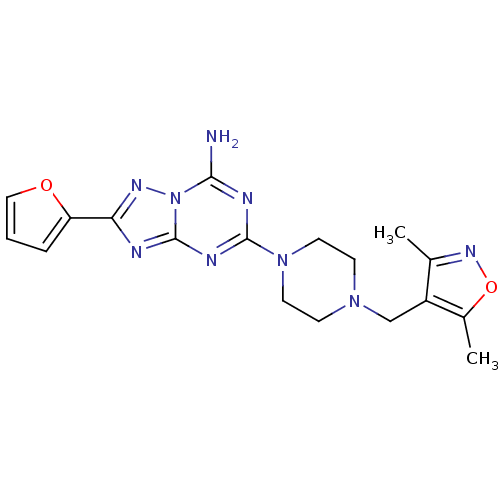
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Vu, CB; Peng, B; Kumaravel, G; Smits, G; Jin, X; Phadke, D; Engber, T; Huang, C; Reilly, J; Tam, S; Grant, D; Hetu, G; Chen, L; Zhang, J; Petter, RC Piperazine derivatives of [1,2,4]triazolo[1,5-a][1,3,5]triazine as potent and selective adenosine A2a receptor antagonists. J Med Chem47:4291-9 (2004) [PubMed] Article
Vu, CB; Peng, B; Kumaravel, G; Smits, G; Jin, X; Phadke, D; Engber, T; Huang, C; Reilly, J; Tam, S; Grant, D; Hetu, G; Chen, L; Zhang, J; Petter, RC Piperazine derivatives of [1,2,4]triazolo[1,5-a][1,3,5]triazine as potent and selective adenosine A2a receptor antagonists. J Med Chem47:4291-9 (2004) [PubMed] Article