| Reaction Details |
|---|
 | Report a problem with these data |
| Target | 5-hydroxytryptamine receptor 1A |
|---|
| Ligand | BDBM50152013 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEBML_306315 |
|---|
| IC50 | 0.700000±n/a nM |
|---|
| Citation |  Heinrich, T; Böttcher, H; Gericke, R; Bartoszyk, GD; Anzali, S; Seyfried, CA; Greiner, HE; Van Amsterdam, C Synthesis and structure--activity relationship in a class of indolebutylpiperazines as dual 5-HT(1A) receptor agonists and serotonin reuptake inhibitors. J Med Chem47:4684-92 (2004) [PubMed] Article Heinrich, T; Böttcher, H; Gericke, R; Bartoszyk, GD; Anzali, S; Seyfried, CA; Greiner, HE; Van Amsterdam, C Synthesis and structure--activity relationship in a class of indolebutylpiperazines as dual 5-HT(1A) receptor agonists and serotonin reuptake inhibitors. J Med Chem47:4684-92 (2004) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| 5-hydroxytryptamine receptor 1A |
|---|
| Name: | 5-hydroxytryptamine receptor 1A |
|---|
| Synonyms: | 5-HT-1A | 5-HT1 | 5-HT1A | 5-Hydroxytryptamine receptor 1A (5-HT1A) | 5-hydroxytryptamine receptor 1A (5HT1A) | 5HT1A_RAT | 5ht1a | G-21 | Htr1a | Serotonin 1 (5-HT1) receptor | Serotonin 1a (5-HT1a) receptor/Adrenergic receptor alpha-1 | Serotonin receptor 1A |
|---|
| Type: | G Protein-Coupled Receptor (GPCR) |
|---|
| Mol. Mass.: | 46445.29 |
|---|
| Organism: | Rattus norvegicus (rat) |
|---|
| Description: | Binding assays were performed using rat hippocampal membranes. |
|---|
| Residue: | 422 |
|---|
| Sequence: | MDVFSFGQGNNTTASQEPFGTGGNVTSISDVTFSYQVITSLLLGTLIFCAVLGNACVVAA
IALERSLQNVANYLIGSLAVTDLMVSVLVLPMAALYQVLNKWTLGQVTCDLFIALDVLCC
TSSILHLCAIALDRYWAITDPIDYVNKRTPRRAAALISLTWLIGFLISIPPMLGWRTPED
RSDPDACTISKDHGYTIYSTFGAFYIPLLLMLVLYGRIFRAARFRIRKTVRKVEKKGAGT
SLGTSSAPPPKKSLNGQPGSGDWRRCAENRAVGTPCTNGAVRQGDDEATLEVIEVHRVGN
SKEHLPLPSESGSNSYAPACLERKNERNAEAKRKMALARERKTVKTLGIIMGTFILCWLP
FFIVALVLPFCESSCHMPALLGAIINWLGYSNSLLNPVIYAYFNKDFQNAFKKIIKCKFC
RR
|
|
|
|---|
| BDBM50152013 |
|---|
| n/a |
|---|
| Name | BDBM50152013 |
|---|
| Synonyms: | 6-{4-[4-(5-Fluoro-1H-indol-3-yl)-butyl]-piperazin-1-yl}-chromen-2-one | CHEMBL278471 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C25H26FN3O2 |
|---|
| Mol. Mass. | 419.4912 |
|---|
| SMILES | Fc1ccc2[nH]cc(CCCCN3CCN(CC3)c3ccc4oc(=O)ccc4c3)c2c1 |
|---|
| Structure | 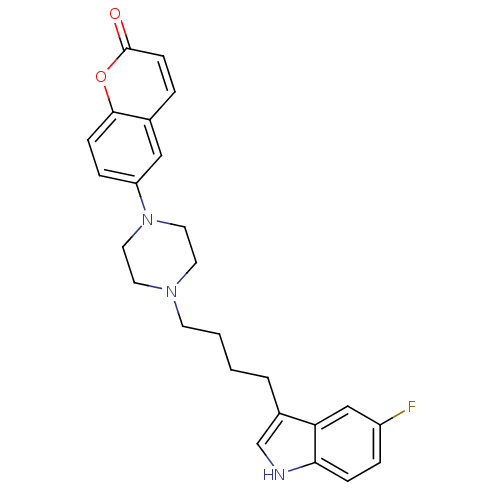
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Heinrich, T; Böttcher, H; Gericke, R; Bartoszyk, GD; Anzali, S; Seyfried, CA; Greiner, HE; Van Amsterdam, C Synthesis and structure--activity relationship in a class of indolebutylpiperazines as dual 5-HT(1A) receptor agonists and serotonin reuptake inhibitors. J Med Chem47:4684-92 (2004) [PubMed] Article
Heinrich, T; Böttcher, H; Gericke, R; Bartoszyk, GD; Anzali, S; Seyfried, CA; Greiner, HE; Van Amsterdam, C Synthesis and structure--activity relationship in a class of indolebutylpiperazines as dual 5-HT(1A) receptor agonists and serotonin reuptake inhibitors. J Med Chem47:4684-92 (2004) [PubMed] Article