| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Histamine H1 receptor |
|---|
| Ligand | BDBM50156874 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_429751 (CHEMBL914451) |
|---|
| IC50 | 150±n/a nM |
|---|
| Citation |  Fonquerna, S; Miralpeix, M; Pagès, L; Puig, C; Cardús, A; Antón, F; Cárdenas, A; Vilella, D; Aparici, M; Calaf, E; Prieto, J; Gras, J; Huerta, JM; Warrellow, G; Beleta, J; Ryder, H Synthesis and structure-activity relationships of novel histamine H1 antagonists: indolylpiperidinyl benzoic acid derivatives. J Med Chem47:6326-37 (2004) [PubMed] Article Fonquerna, S; Miralpeix, M; Pagès, L; Puig, C; Cardús, A; Antón, F; Cárdenas, A; Vilella, D; Aparici, M; Calaf, E; Prieto, J; Gras, J; Huerta, JM; Warrellow, G; Beleta, J; Ryder, H Synthesis and structure-activity relationships of novel histamine H1 antagonists: indolylpiperidinyl benzoic acid derivatives. J Med Chem47:6326-37 (2004) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Histamine H1 receptor |
|---|
| Name: | Histamine H1 receptor |
|---|
| Synonyms: | HISTAMINE H1 | HRH1 | HRH1_CAVPO |
|---|
| Type: | G Protein-Coupled Receptor (GPCR) |
|---|
| Mol. Mass.: | 55641.53 |
|---|
| Organism: | Cavia porcellus (domestic guinea pig) |
|---|
| Description: | Guinea pig cerebellum was used in binding assay. |
|---|
| Residue: | 488 |
|---|
| Sequence: | MSFLPGMTPVTLSNFSWALEDRMLEGNSTTTPTRQLMPLVVVLSSVSLVTVALNLLVLYA
VRSERKLHTVGNLYIVSLSVADLIVGAVVMPMSILYLHRSAWILGRPLCLFWLSMDYVAS
TASIFSVFILCIDRYRSVQQPLRYLRYRTKTRASATILGAWLLSFLWVIPILGWHHFMAP
TSEPREKKCETDFYDVTWFKVMTAIINFYLPTLLMLWFYIRIYKAVRRHCQHRQLINSSL
PSFSEMKLKLENAKVDTRRMGKESPWEDPKRCSKDASGVHTPMPSSQHLVDMPCAAVLSE
DEGGEVGTRQMPMLAVGDGRCCEALNHMHSQLELSGQSRATHSISARPEEWTVVDGQSFP
ITDSDTSTEAAPMGGQPRSGSNSGLDYIKFTWRRLRSHSRQYTSGLHLNRERKAAKQLGC
IMAAFILCWIPYFVFFMVIAFCKSCSNEPVHMFTIWLGYLNSTLNPLIYPLCNENFRKTF
KRILRIPP
|
|
|
|---|
| BDBM50156874 |
|---|
| n/a |
|---|
| Name | BDBM50156874 |
|---|
| Synonyms: | 5-{4-[1-(5-chlorothiophen-2-ylmethyl)-6-fluoro-1H-indol-3-yl]piperidin-1-ylmethyl}-2-methoxybenzoic acid | CHEMBL221730 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C27H26ClFN2O3S |
|---|
| Mol. Mass. | 513.023 |
|---|
| SMILES | COc1ccc(CN2CCC(CC2)c2cn(Cc3ccc(Cl)s3)c3cc(F)ccc23)cc1C(O)=O |
|---|
| Structure | 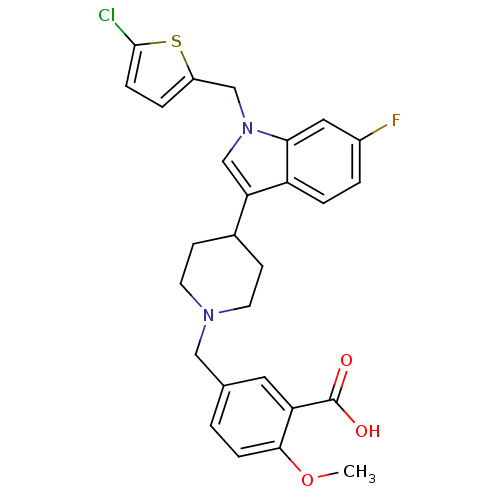
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Fonquerna, S; Miralpeix, M; Pagès, L; Puig, C; Cardús, A; Antón, F; Cárdenas, A; Vilella, D; Aparici, M; Calaf, E; Prieto, J; Gras, J; Huerta, JM; Warrellow, G; Beleta, J; Ryder, H Synthesis and structure-activity relationships of novel histamine H1 antagonists: indolylpiperidinyl benzoic acid derivatives. J Med Chem47:6326-37 (2004) [PubMed] Article
Fonquerna, S; Miralpeix, M; Pagès, L; Puig, C; Cardús, A; Antón, F; Cárdenas, A; Vilella, D; Aparici, M; Calaf, E; Prieto, J; Gras, J; Huerta, JM; Warrellow, G; Beleta, J; Ryder, H Synthesis and structure-activity relationships of novel histamine H1 antagonists: indolylpiperidinyl benzoic acid derivatives. J Med Chem47:6326-37 (2004) [PubMed] Article