| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Muscarinic acetylcholine receptor M3 |
|---|
| Ligand | BDBM50165018 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_302413 (CHEMBL828817) |
|---|
| Ki | 50±n/a nM |
|---|
| Citation |  Kaur, K; Aeron, S; Bruhaspathy, M; Shetty, SJ; Gupta, S; Hegde, LH; Silamkoti, AD; Mehta, A; Chugh, A; Gupta, JB; Sarma, PK; Kumar, N Design, synthesis and activity of novel derivatives of oxybutynin and tolterodine. Bioorg Med Chem Lett15:2093-6 (2005) [PubMed] Article Kaur, K; Aeron, S; Bruhaspathy, M; Shetty, SJ; Gupta, S; Hegde, LH; Silamkoti, AD; Mehta, A; Chugh, A; Gupta, JB; Sarma, PK; Kumar, N Design, synthesis and activity of novel derivatives of oxybutynin and tolterodine. Bioorg Med Chem Lett15:2093-6 (2005) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Muscarinic acetylcholine receptor M3 |
|---|
| Name: | Muscarinic acetylcholine receptor M3 |
|---|
| Synonyms: | ACM3_RAT | Cholinergic, muscarinic M3 | Chrm-3 | Chrm3 |
|---|
| Type: | Enzyme Catalytic Domain |
|---|
| Mol. Mass.: | 66086.66 |
|---|
| Organism: | RAT |
|---|
| Description: | Cholinergic, muscarinic M3 CHRM3 RAT::P08483 |
|---|
| Residue: | 589 |
|---|
| Sequence: | MTLHSNSTTSPLFPNISSSWVHSPSEAGLPLGTVTQLGSYNISQETGNFSSNDTSSDPLG
GHTIWQVVFIAFLTGFLALVTIIGNILVIVAFKVNKQLKTVNNYFLLSLACADLIIGVIS
MNLFTTYIIMNRWALGNLACDLWLSIDYVASNASVMNLLVISFDRYFSITRPLTYRAKRT
TKRAGVMIGLAWVISFVLWAPAILFWQYFVGKRTVPPGECFIQFLSEPTITFGTAIAAFY
MPVTIMTILYWRIYKETEKRTKELAGLQASGTEAEAENFVHPTGSSRSCSSYELQQQGVK
RSSRRKYGRCHFWFTTKSWKPSAEQMDQDHSSSDSWNNNDAAASLENSASSDEEDIGSET
RAIYSIVLKLPGHSSILNSTKLPSSDNLQVSNEDLGTVDVERNAHKLQAQKSMGDGDNCQ
KDFTKLPIQLESAVDTGKTSDTNSSADKTTATLPLSFKEATLAKRFALKTRSQITKRKRM
SLIKEKKAAQTLSAILLAFIITWTPYNIMVLVNTFCDSCIPKTYWNLGYWLCYINSTVNP
VCYALCNKTFRTTFKTLLLCQCDKRKRRKQQYQQRQSVIFHKRVPEQAL
|
|
|
|---|
| BDBM50165018 |
|---|
| n/a |
|---|
| Name | BDBM50165018 |
|---|
| Synonyms: | 2-((1R,5S)-3-3-Aza-bicyclo[3.1.0]hex-3-yl-1-phenyl-propyl)-4-methyl-phenol | CHEMBL190815 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C21H25NO |
|---|
| Mol. Mass. | 307.4293 |
|---|
| SMILES | Cc1ccc(O)c(c1)C(CCN1C[C@@H]2C[C@@H]2C1)c1ccccc1 |
|---|
| Structure | 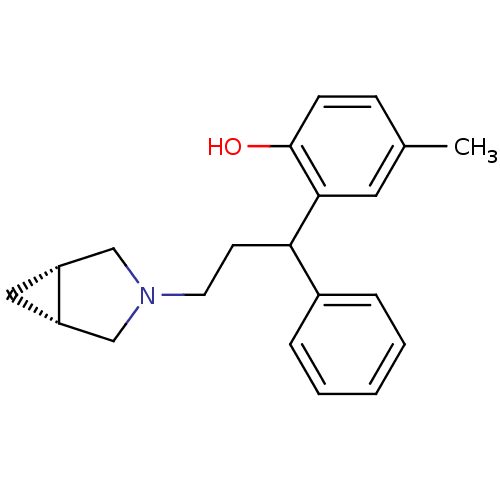
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Kaur, K; Aeron, S; Bruhaspathy, M; Shetty, SJ; Gupta, S; Hegde, LH; Silamkoti, AD; Mehta, A; Chugh, A; Gupta, JB; Sarma, PK; Kumar, N Design, synthesis and activity of novel derivatives of oxybutynin and tolterodine. Bioorg Med Chem Lett15:2093-6 (2005) [PubMed] Article
Kaur, K; Aeron, S; Bruhaspathy, M; Shetty, SJ; Gupta, S; Hegde, LH; Silamkoti, AD; Mehta, A; Chugh, A; Gupta, JB; Sarma, PK; Kumar, N Design, synthesis and activity of novel derivatives of oxybutynin and tolterodine. Bioorg Med Chem Lett15:2093-6 (2005) [PubMed] Article