| Reaction Details |
|---|
 | Report a problem with these data |
| Target | RNA-directed RNA polymerase |
|---|
| Ligand | BDBM50169927 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_305448 (CHEMBL830123) |
|---|
| IC50 | 31±n/a nM |
|---|
| Citation |  Harper, S; Avolio, S; Pacini, B; Di Filippo, M; Altamura, S; Tomei, L; Paonessa, G; Di Marco, S; Carfi, A; Giuliano, C; Padron, J; Bonelli, F; Migliaccio, G; De Francesco, R; Laufer, R; Rowley, M; Narjes, F Potent inhibitors of subgenomic hepatitis C virus RNA replication through optimization of indole-N-acetamide allosteric inhibitors of the viral NS5B polymerase. J Med Chem48:4547-57 (2005) [PubMed] Article Harper, S; Avolio, S; Pacini, B; Di Filippo, M; Altamura, S; Tomei, L; Paonessa, G; Di Marco, S; Carfi, A; Giuliano, C; Padron, J; Bonelli, F; Migliaccio, G; De Francesco, R; Laufer, R; Rowley, M; Narjes, F Potent inhibitors of subgenomic hepatitis C virus RNA replication through optimization of indole-N-acetamide allosteric inhibitors of the viral NS5B polymerase. J Med Chem48:4547-57 (2005) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| RNA-directed RNA polymerase |
|---|
| Name: | RNA-directed RNA polymerase |
|---|
| Synonyms: | Hepatitis C virus NS5B RNA-dependent RNA polymerase | NS5B protein |
|---|
| Type: | Protein |
|---|
| Mol. Mass.: | 25173.95 |
|---|
| Organism: | Hepatitis C virus |
|---|
| Description: | Q8JXU8 |
|---|
| Residue: | 229 |
|---|
| Sequence: | RTEEAIYQCCDLDPQARVAIRSLTERLYVGGPLTNSRGENCGYRRRASGVLTTSCGNTLT
CYIKAQAACRAAGRQDCTMLVCGDDLVVICESAGVQEDAASLRAFTEAMTRYSAPPGDPP
QPEYDLELITSCSSNVSVAHDGAGKRVYYLTRDPTTPLARAAWETARHTPVNSWLGNIIM
FAPTLWVRMIMLTHFFSVLIARDQLEQALDCEIYGACYSIEPLLPPIIQ
|
|
|
|---|
| BDBM50169927 |
|---|
| n/a |
|---|
| Name | BDBM50169927 |
|---|
| Synonyms: | 3-Cyclohexyl-1-dimethylcarbamoylmethyl-2-furan-3-yl-1H-indole-6-carboxylic acid | 3-cyclohexyl-1-(2-(dimethylamino)-2-oxoethyl)-2-(furan-3-yl)-1H-indole-6-carboxylic acid | CHEMBL190558 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C23H26N2O4 |
|---|
| Mol. Mass. | 394.4635 |
|---|
| SMILES | CN(C)C(=O)Cn1c(-c2ccoc2)c(C2CCCCC2)c2ccc(cc12)C(O)=O |
|---|
| Structure | 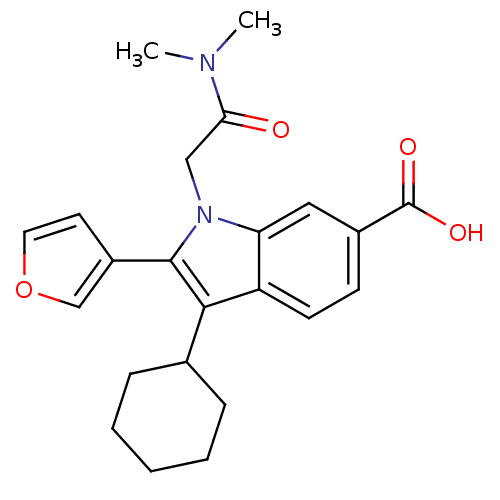
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Harper, S; Avolio, S; Pacini, B; Di Filippo, M; Altamura, S; Tomei, L; Paonessa, G; Di Marco, S; Carfi, A; Giuliano, C; Padron, J; Bonelli, F; Migliaccio, G; De Francesco, R; Laufer, R; Rowley, M; Narjes, F Potent inhibitors of subgenomic hepatitis C virus RNA replication through optimization of indole-N-acetamide allosteric inhibitors of the viral NS5B polymerase. J Med Chem48:4547-57 (2005) [PubMed] Article
Harper, S; Avolio, S; Pacini, B; Di Filippo, M; Altamura, S; Tomei, L; Paonessa, G; Di Marco, S; Carfi, A; Giuliano, C; Padron, J; Bonelli, F; Migliaccio, G; De Francesco, R; Laufer, R; Rowley, M; Narjes, F Potent inhibitors of subgenomic hepatitis C virus RNA replication through optimization of indole-N-acetamide allosteric inhibitors of the viral NS5B polymerase. J Med Chem48:4547-57 (2005) [PubMed] Article