| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Hepatocyte growth factor receptor |
|---|
| Ligand | BDBM13923 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_321400 (CHEMBL880635) |
|---|
| IC50 | >10000±n/a nM |
|---|
| Citation |  Fink, BE; Vite, GD; Mastalerz, H; Kadow, JF; Kim, SH; Leavitt, KJ; Du, K; Crews, D; Mitt, T; Wong, TW; Hunt, JT; Vyas, DM; Tokarski, JS New dual inhibitors of EGFR and HER2 protein tyrosine kinases. Bioorg Med Chem Lett15:4774-9 (2005) [PubMed] Article Fink, BE; Vite, GD; Mastalerz, H; Kadow, JF; Kim, SH; Leavitt, KJ; Du, K; Crews, D; Mitt, T; Wong, TW; Hunt, JT; Vyas, DM; Tokarski, JS New dual inhibitors of EGFR and HER2 protein tyrosine kinases. Bioorg Med Chem Lett15:4774-9 (2005) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Hepatocyte growth factor receptor |
|---|
| Name: | Hepatocyte growth factor receptor |
|---|
| Synonyms: | Hepatocyte growth factor receptor | Hepatocyte growth factor receptor (MET) | Hepatocyte growth factor receptor (c-MET) | Hepatocyte growth factor receptor (cMET) | MET | MET_HUMAN | Met proto-oncogene (hepatocyte growth factor receptor) | Proto-oncogene c-Met | Tyrosine-protein kinase Met (c-Met) | Tyrosine-protein kinase Met (cMet) | c-Met kinase |
|---|
| Type: | Protein |
|---|
| Mol. Mass.: | 155559.73 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | P08581 |
|---|
| Residue: | 1390 |
|---|
| Sequence: | MKAPAVLAPGILVLLFTLVQRSNGECKEALAKSEMNVNMKYQLPNFTAETPIQNVILHEH
HIFLGATNYIYVLNEEDLQKVAEYKTGPVLEHPDCFPCQDCSSKANLSGGVWKDNINMAL
VVDTYYDDQLISCGSVNRGTCQRHVFPHNHTADIQSEVHCIFSPQIEEPSQCPDCVVSAL
GAKVLSSVKDRFINFFVGNTINSSYFPDHPLHSISVRRLKETKDGFMFLTDQSYIDVLPE
FRDSYPIKYVHAFESNNFIYFLTVQRETLDAQTFHTRIIRFCSINSGLHSYMEMPLECIL
TEKRKKRSTKKEVFNILQAAYVSKPGAQLARQIGASLNDDILFGVFAQSKPDSAEPMDRS
AMCAFPIKYVNDFFNKIVNKNNVRCLQHFYGPNHEHCFNRTLLRNSSGCEARRDEYRTEF
TTALQRVDLFMGQFSEVLLTSISTFIKGDLTIANLGTSEGRFMQVVVSRSGPSTPHVNFL
LDSHPVSPEVIVEHTLNQNGYTLVITGKKITKIPLNGLGCRHFQSCSQCLSAPPFVQCGW
CHDKCVRSEECLSGTWTQQICLPAIYKVFPNSAPLEGGTRLTICGWDFGFRRNNKFDLKK
TRVLLGNESCTLTLSESTMNTLKCTVGPAMNKHFNMSIIISNGHGTTQYSTFSYVDPVIT
SISPKYGPMAGGTLLTLTGNYLNSGNSRHISIGGKTCTLKSVSNSILECYTPAQTISTEF
AVKLKIDLANRETSIFSYREDPIVYEIHPTKSFISGGSTITGVGKNLNSVSVPRMVINVH
EAGRNFTVACQHRSNSEIICCTTPSLQQLNLQLPLKTKAFFMLDGILSKYFDLIYVHNPV
FKPFEKPVMISMGNENVLEIKGNDIDPEAVKGEVLKVGNKSCENIHLHSEAVLCTVPNDL
LKLNSELNIEWKQAISSTVLGKVIVQPDQNFTGLIAGVVSISTALLLLLGFFLWLKKRKQ
IKDLGSELVRYDARVHTPHLDRLVSARSVSPTTEMVSNESVDYRATFPEDQFPNSSQNGS
CRQVQYPLTDMSPILTSGDSDISSPLLQNTVHIDLSALNPELVQAVQHVVIGPSSLIVHF
NEVIGRGHFGCVYHGTLLDNDGKKIHCAVKSLNRITDIGEVSQFLTEGIIMKDFSHPNVL
SLLGICLRSEGSPLVVLPYMKHGDLRNFIRNETHNPTVKDLIGFGLQVAKGMKYLASKKF
VHRDLAARNCMLDEKFTVKVADFGLARDMYDKEYYSVHNKTGAKLPVKWMALESLQTQKF
TTKSDVWSFGVLLWELMTRGAPPYPDVNTFDITVYLLQGRRLLQPEYCPDPLYEVMLKCW
HPKAEMRPSFSELVSRISAIFSTFIGEHYVHVNATYVNVKCVAPYPSLLSSEDNADDEVD
TRPASFWETS
|
|
|
|---|
| BDBM13923 |
|---|
| n/a |
|---|
| Name | BDBM13923 |
|---|
| Synonyms: | 2-(1H-imidazol-1-yl)ethyl N-{4-[(1-benzyl-1H-indazol-5-yl)amino]-5-ethylpyrrolo[1,2-a][1,2,4]triazin-6-yl}carbamate | CHEMBL412367 | pyrrolo[2,1-f][1,2,4]triazine analog 9b |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C28H27N9O2 |
|---|
| Mol. Mass. | 521.5731 |
|---|
| SMILES | CCc1c(NC(=O)OCCn2ccnc2)cn2ncnc(Nc3ccc4n(Cc5ccccc5)ncc4c3)c12 |
|---|
| Structure | 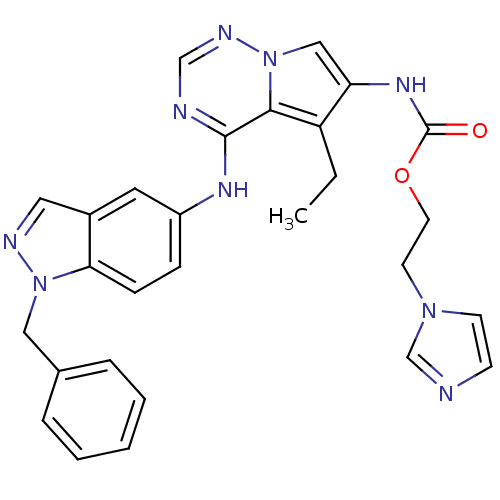
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Fink, BE; Vite, GD; Mastalerz, H; Kadow, JF; Kim, SH; Leavitt, KJ; Du, K; Crews, D; Mitt, T; Wong, TW; Hunt, JT; Vyas, DM; Tokarski, JS New dual inhibitors of EGFR and HER2 protein tyrosine kinases. Bioorg Med Chem Lett15:4774-9 (2005) [PubMed] Article
Fink, BE; Vite, GD; Mastalerz, H; Kadow, JF; Kim, SH; Leavitt, KJ; Du, K; Crews, D; Mitt, T; Wong, TW; Hunt, JT; Vyas, DM; Tokarski, JS New dual inhibitors of EGFR and HER2 protein tyrosine kinases. Bioorg Med Chem Lett15:4774-9 (2005) [PubMed] Article