| Reaction Details |
|---|
 | Report a problem with these data |
| Target | 5-hydroxytryptamine receptor 1A |
|---|
| Ligand | BDBM50130152 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_349600 (CHEMBL866277) |
|---|
| Ki | 5.52±n/a nM |
|---|
| Citation |  Takeuchi, K; Kohn, TJ; Honigschmidt, NA; Rocco, VP; Spinazze, PG; Hemrick-Luecke, SK; Thompson, LK; Evans, DC; Rasmussen, K; Koger, D; Lodge, D; Martin, LJ; Shaw, J; Threlkeld, PG; Wong, DT Advances toward new antidepressants beyond SSRIs: 1-aryloxy-3-piperidinylpropan-2-ols with dual 5-HT1A receptor antagonism/SSRI activities. Part 5. Bioorg Med Chem Lett16:2347-51 (2006) [PubMed] Article Takeuchi, K; Kohn, TJ; Honigschmidt, NA; Rocco, VP; Spinazze, PG; Hemrick-Luecke, SK; Thompson, LK; Evans, DC; Rasmussen, K; Koger, D; Lodge, D; Martin, LJ; Shaw, J; Threlkeld, PG; Wong, DT Advances toward new antidepressants beyond SSRIs: 1-aryloxy-3-piperidinylpropan-2-ols with dual 5-HT1A receptor antagonism/SSRI activities. Part 5. Bioorg Med Chem Lett16:2347-51 (2006) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| 5-hydroxytryptamine receptor 1A |
|---|
| Name: | 5-hydroxytryptamine receptor 1A |
|---|
| Synonyms: | 5-HT-1A | 5-HT1A | 5-hydroxytryptamine receptor 1A (5-HT-1A) | 5HT1A_HUMAN | ADRB2RL1 | ADRBRL1 | Dopamine D2 receptor and serotonin 1a receptor | G-21 | HTR1A | Serotonin receptor 1A |
|---|
| Type: | n/a |
|---|
| Mol. Mass.: | 46122.49 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | n/a |
|---|
| Residue: | 422 |
|---|
| Sequence: | MDVLSPGQGNNTTSPPAPFETGGNTTGISDVTVSYQVITSLLLGTLIFCAVLGNACVVAA
IALERSLQNVANYLIGSLAVTDLMVSVLVLPMAALYQVLNKWTLGQVTCDLFIALDVLCC
TSSILHLCAIALDRYWAITDPIDYVNKRTPRRAAALISLTWLIGFLISIPPMLGWRTPED
RSDPDACTISKDHGYTIYSTFGAFYIPLLLMLVLYGRIFRAARFRIRKTVKKVEKTGADT
RHGASPAPQPKKSVNGESGSRNWRLGVESKAGGALCANGAVRQGDDGAALEVIEVHRVGN
SKEHLPLPSEAGPTPCAPASFERKNERNAEAKRKMALARERKTVKTLGIIMGTFILCWLP
FFIVALVLPFCESSCHMPTLLGAIINWLGYSNSLLNPVIYAYFNKDFQNAFKKIIKCKFC
RQ
|
|
|
|---|
| BDBM50130152 |
|---|
| n/a |
|---|
| Name | BDBM50130152 |
|---|
| Synonyms: | (S)-1-(1H-indol-4-yloxy)-3-((2S,4R)-4-(6-fluorobenzo[b]thiophen-2-yl)-2-methylpiperidin-1-yl)propan-2-ol | (S)-1-[(4S,6R)-4-(6-Fluoro-benzo[b]thiophen-2-yl)-2-methyl-piperidin-1-yl]-3-(1H-indol-4-yloxy)-propan-2-ol | CHEMBL309396 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C25H27FN2O2S |
|---|
| Mol. Mass. | 438.557 |
|---|
| SMILES | C[C@H]1C[C@@H](CCN1C[C@H](O)COc1cccc2[nH]ccc12)c1cc2ccc(F)cc2s1 |
|---|
| Structure | 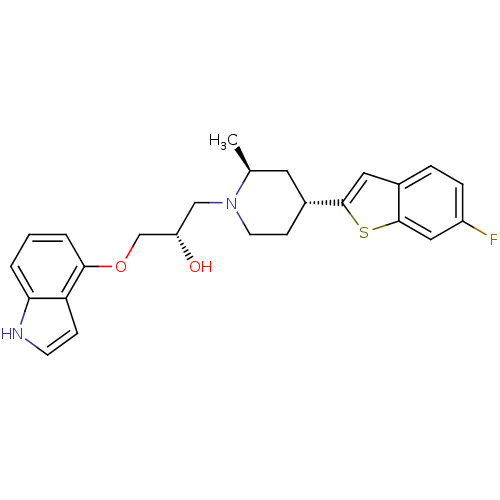
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Takeuchi, K; Kohn, TJ; Honigschmidt, NA; Rocco, VP; Spinazze, PG; Hemrick-Luecke, SK; Thompson, LK; Evans, DC; Rasmussen, K; Koger, D; Lodge, D; Martin, LJ; Shaw, J; Threlkeld, PG; Wong, DT Advances toward new antidepressants beyond SSRIs: 1-aryloxy-3-piperidinylpropan-2-ols with dual 5-HT1A receptor antagonism/SSRI activities. Part 5. Bioorg Med Chem Lett16:2347-51 (2006) [PubMed] Article
Takeuchi, K; Kohn, TJ; Honigschmidt, NA; Rocco, VP; Spinazze, PG; Hemrick-Luecke, SK; Thompson, LK; Evans, DC; Rasmussen, K; Koger, D; Lodge, D; Martin, LJ; Shaw, J; Threlkeld, PG; Wong, DT Advances toward new antidepressants beyond SSRIs: 1-aryloxy-3-piperidinylpropan-2-ols with dual 5-HT1A receptor antagonism/SSRI activities. Part 5. Bioorg Med Chem Lett16:2347-51 (2006) [PubMed] Article