

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Reaction Details | |||
|---|---|---|---|
 | Report a problem with these data | ||
| Target | Substance-P receptor | ||
| Ligand | BDBM50179775 | ||
| Substrate/Competitor | n/a | ||
| Meas. Tech. | ChEMBL_336032 (CHEMBL867164) | ||
| IC50 | 0.48±n/a nM | ||
| Citation |  Hollingworth, GJ; Carlson, EJ; Castro, JL; Chicchi, GG; Clark, N; Cooper, LC; Dirat, O; Salvo, JD; Elliott, JM; Kilburn, R; Kurtz, MM; Rycroft, W; Tattersall, FD; Tsao, KL; Swain, CJ Novel lactam NK1 antagonists with anti-emetic activity. Bioorg Med Chem Lett16:1197-201 (2006) [PubMed] Article Hollingworth, GJ; Carlson, EJ; Castro, JL; Chicchi, GG; Clark, N; Cooper, LC; Dirat, O; Salvo, JD; Elliott, JM; Kilburn, R; Kurtz, MM; Rycroft, W; Tattersall, FD; Tsao, KL; Swain, CJ Novel lactam NK1 antagonists with anti-emetic activity. Bioorg Med Chem Lett16:1197-201 (2006) [PubMed] Article | ||
| More Info.: | Get all data from this article, Assay Method | ||
| Substance-P receptor | |||
| Name: | Substance-P receptor | ||
| Synonyms: | NK-1 receptor | NK-1R | NK1 Receptor | NK1R | NK1R_HUMAN | Neurokinin 1 receptor | Neurokinin-1 (NK-1) | Neuromedin-1 receptor (NK-1R) | SPR | TAC1R | TACR1 | Tachykinin receptor 1 | Tachykinin receptor 1 (NK1) | tachykinin | ||
| Type: | G Protein-Coupled Receptor (GPCR) | ||
| Mol. Mass.: | 46254.43 | ||
| Organism: | Homo sapiens (Human) | ||
| Description: | P25103 | ||
| Residue: | 407 | ||
| Sequence: |
| ||
| BDBM50179775 | |||
| n/a | |||
| Name | BDBM50179775 | ||
| Synonyms: | 4-((1r,4r)-4-(3-(3,5-bis(trifluoromethyl)phenyl)-3-methyl-2-oxopyrrolidin-1-yl)-4-phenylcyclohexyl)-1-(2-chlorophenyl)piperazin-2-one | CHEMBL381984 | ||
| Type | Small organic molecule | ||
| Emp. Form. | C35H34ClF6N3O2 | ||
| Mol. Mass. | 678.107 | ||
| SMILES | CC1(CCN(C1=O)[C@]1(CC[C@@H](CC1)N1CCN(C(=O)C1)c1ccccc1Cl)c1ccccc1)c1cc(cc(c1)C(F)(F)F)C(F)(F)F |wU:7.7,wD:10.14,(21.77,-10.25,;21.01,-11.59,;20.37,-10.19,;18.84,-10.36,;18.53,-11.87,;19.87,-12.63,;20.05,-14.16,;17.21,-12.65,;18.54,-13.42,;18.54,-14.96,;17.21,-15.73,;15.88,-14.96,;15.88,-13.42,;17.21,-17.27,;15.87,-18.04,;15.87,-19.59,;17.21,-20.35,;18.54,-19.58,;19.88,-20.34,;18.54,-18.04,;17.22,-21.89,;15.89,-22.66,;15.89,-24.2,;17.23,-24.97,;18.56,-24.19,;18.55,-22.65,;19.88,-21.87,;15.87,-11.88,;15.87,-10.33,;14.52,-9.56,;13.18,-10.33,;13.19,-11.89,;14.54,-12.66,;22.52,-11.9,;23.84,-11.11,;25.18,-11.86,;25.19,-13.41,;23.86,-14.19,;22.52,-13.43,;23.87,-15.73,;23.86,-17.26,;25.41,-15.73,;22.33,-15.74,;26.5,-11.08,;27.82,-10.3,;25.72,-9.75,;27.29,-12.4,)| | ||
| Structure | 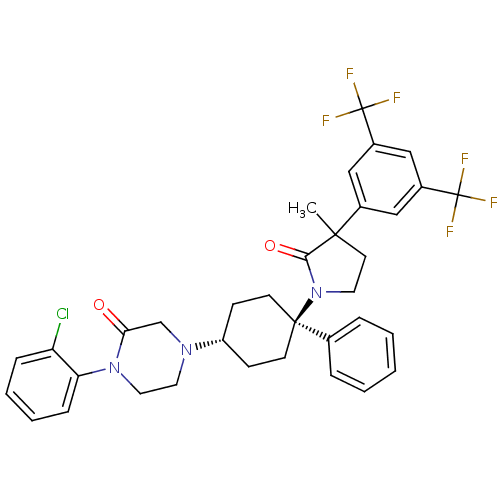
| ||