| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Reverse transcriptase/RNaseH |
|---|
| Ligand | BDBM50183217 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_355438 (CHEMBL868998) |
|---|
| IC50 | 3±n/a nM |
|---|
| Citation |  Muraglia, E; Kinzel, OD; Laufer, R; Miller, MD; Moyer, G; Munshi, V; Orvieto, F; Palumbi, MC; Pescatore, G; Rowley, M; Williams, PD; Summa, V Tetrazole thioacetanilides: potent non-nucleoside inhibitors of WT HIV reverse transcriptase and its K103N mutant. Bioorg Med Chem Lett16:2748-52 (2006) [PubMed] Article Muraglia, E; Kinzel, OD; Laufer, R; Miller, MD; Moyer, G; Munshi, V; Orvieto, F; Palumbi, MC; Pescatore, G; Rowley, M; Williams, PD; Summa, V Tetrazole thioacetanilides: potent non-nucleoside inhibitors of WT HIV reverse transcriptase and its K103N mutant. Bioorg Med Chem Lett16:2748-52 (2006) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Reverse transcriptase/RNaseH |
|---|
| Name: | Reverse transcriptase/RNaseH |
|---|
| Synonyms: | HIV-1 Reverse Transcriptase RNase H | Human immunodeficiency virus type 1 reverse transcriptase | Reverse transcriptase/RNaseH |
|---|
| Type: | PROTEIN |
|---|
| Mol. Mass.: | 65229.15 |
|---|
| Organism: | Human immunodeficiency virus 1 |
|---|
| Description: | ChEMBL_1473730 |
|---|
| Residue: | 566 |
|---|
| Sequence: | PISPIETVPVKLKPGMDGPKVKQWPLTEEKIKALVEICTEMEKEGKISKIGPENPYNTPV
FAIKKKDSTKWRKLVDFRELNKRTQDFWEVQLGIPHPAGLKKRKSVTVLDVGDAYFSVPL
DEDFRKYTAFTIPSINNETPGIRYQYNVLPQGWKGSPAIFQSSMTKILEPFRKQNPDIVI
YQYMDDLYVGSDLEIGQHRTKIEELRQHLLRWGLTTPDKKHQKEPPFLWMGYELHPDKWT
VQPIVLPEKDSWTVNDIQKLVGKLNWASQIYPGIRVRQLCKLLRGTKALTEVIPLTEEAE
LELAENREILKEPVHGVYYDPSKDLIAEIQKQGQGQWTYQIYQEPFKNLRTGKYARMRGA
HTNDVKQLTEAVQKITTESIVIWGKTPKFKLPIQKETWETWWTEYWQATWIPEWEFVNTP
PLVKLWYQLEKEPIVGAETFYVDGAANRETKLGKAGYVTNRGRQKVVTLTDTTNQKTELQ
AIYLALQDSGLEVNIVTDSQYALGIIQAQPDQSESELVNQIIEQLIKKEKVYLAWVPAHK
GIGGNEQVDKLVSAGIRKVLFLDGID
|
|
|
|---|
| BDBM50183217 |
|---|
| n/a |
|---|
| Name | BDBM50183217 |
|---|
| Synonyms: | CHEMBL206522 | N-(2-chloro-4-sulfamoyl-phenyl)-2-[1-(2,5-dichloro-phenyl)-1H-tetrazol-5-ylsulfanyl]-acetamide |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C15H11Cl3N6O3S2 |
|---|
| Mol. Mass. | 493.775 |
|---|
| SMILES | NS(=O)(=O)c1ccc(NC(=O)CSc2nnnn2-c2cc(Cl)ccc2Cl)c(Cl)c1 |
|---|
| Structure | 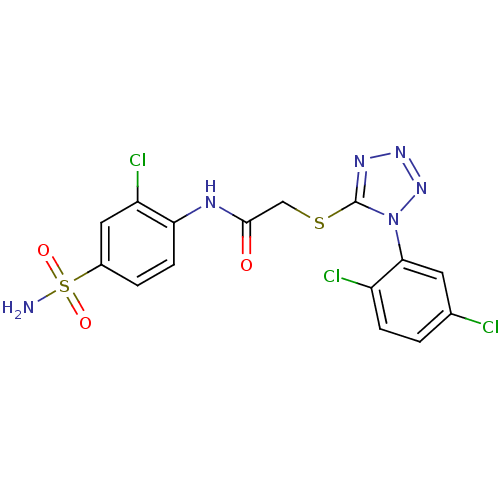
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Muraglia, E; Kinzel, OD; Laufer, R; Miller, MD; Moyer, G; Munshi, V; Orvieto, F; Palumbi, MC; Pescatore, G; Rowley, M; Williams, PD; Summa, V Tetrazole thioacetanilides: potent non-nucleoside inhibitors of WT HIV reverse transcriptase and its K103N mutant. Bioorg Med Chem Lett16:2748-52 (2006) [PubMed] Article
Muraglia, E; Kinzel, OD; Laufer, R; Miller, MD; Moyer, G; Munshi, V; Orvieto, F; Palumbi, MC; Pescatore, G; Rowley, M; Williams, PD; Summa, V Tetrazole thioacetanilides: potent non-nucleoside inhibitors of WT HIV reverse transcriptase and its K103N mutant. Bioorg Med Chem Lett16:2748-52 (2006) [PubMed] Article