| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Sphingosine 1-phosphate receptor 2 |
|---|
| Ligand | BDBM50186926 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_376407 (CHEMBL866633) |
|---|
| EC50 | >10000±n/a nM |
|---|
| Citation |  Yan, L; Huo, P; Doherty, G; Toth, L; Hale, JJ; Mills, SG; Hajdu, R; Keohane, CA; Rosenbach, MJ; Milligan, JA; Shei, GJ; Chrebet, G; Bergstrom, J; Card, D; Quackenbush, E; Wickham, A; Mandala, SM Discovery of 3-arylpropionic acids as potent agonists of sphingosine-1-phosphate receptor-1 (S1P1) with high selectivity against all other known S1P receptor subtypes. Bioorg Med Chem Lett16:3679-83 (2006) [PubMed] Article Yan, L; Huo, P; Doherty, G; Toth, L; Hale, JJ; Mills, SG; Hajdu, R; Keohane, CA; Rosenbach, MJ; Milligan, JA; Shei, GJ; Chrebet, G; Bergstrom, J; Card, D; Quackenbush, E; Wickham, A; Mandala, SM Discovery of 3-arylpropionic acids as potent agonists of sphingosine-1-phosphate receptor-1 (S1P1) with high selectivity against all other known S1P receptor subtypes. Bioorg Med Chem Lett16:3679-83 (2006) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Sphingosine 1-phosphate receptor 2 |
|---|
| Name: | Sphingosine 1-phosphate receptor 2 |
|---|
| Synonyms: | EDG5 | S1P2 | S1PR2 | S1PR2_HUMAN | Sphingosine 1-phosphate receptor | Sphingosine 1-phosphate receptor Edg-5 | Sphingosine-1-phosphate receptor 2 | ndothelial differentiation G-protein coupled receptor 5 |
|---|
| Type: | G Protein-Coupled Receptor (GPCR) |
|---|
| Mol. Mass.: | 38883.16 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | Membranes isolated from S1P2-transfected CHO cells were used in ligand binding assay. |
|---|
| Residue: | 353 |
|---|
| Sequence: | MGSLYSEYLNPNKVQEHYNYTKETLETQETTSRQVASAFIVILCCAIVVENLLVLIAVAR
NSKFHSAMYLFLGNLAASDLLAGVAFVANTLLSGSVTLRLTPVQWFAREGSAFITLSASV
FSLLAIAIERHVAIAKVKLYGSDKSCRMLLLIGASWLISLVLGGLPILGWNCLGHLEACS
TVLPLYAKHYVLCVVTIFSIILLAIVALYVRIYCVVRSSHADMAAPQTLALLKTVTIVLG
VFIVCWLPAFSILLLDYACPVHSCPILYKAHYFFAVSTLNSLLNPVIYTWRSRDLRREVL
RPLQCWRPGVGVQGRRRGGTPGHHLLPLRSSSSLERGMHMPTSPTFLEGNTVV
|
|
|
|---|
| BDBM50186926 |
|---|
| n/a |
|---|
| Name | BDBM50186926 |
|---|
| Synonyms: | 3-(4-(5-(4-ethoxy-3-(trifluoromethyl)phenyl)-1,2,4-oxadiazol-3-yl)-3-methylphenyl)propanoic acid | CHEMBL211689 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C21H19F3N2O4 |
|---|
| Mol. Mass. | 420.3818 |
|---|
| SMILES | CCOc1ccc(cc1C(F)(F)F)-c1nc(no1)-c1ccc(CCC(O)=O)cc1C |
|---|
| Structure | 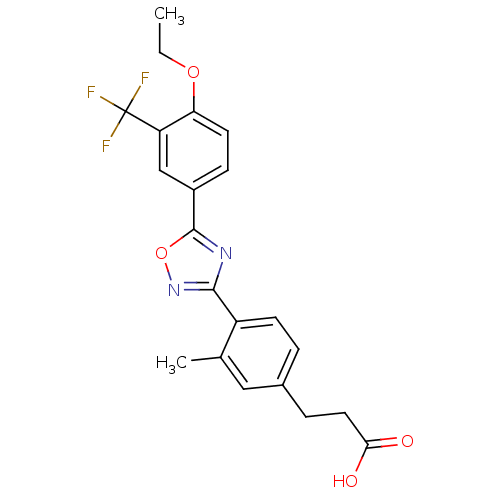
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Yan, L; Huo, P; Doherty, G; Toth, L; Hale, JJ; Mills, SG; Hajdu, R; Keohane, CA; Rosenbach, MJ; Milligan, JA; Shei, GJ; Chrebet, G; Bergstrom, J; Card, D; Quackenbush, E; Wickham, A; Mandala, SM Discovery of 3-arylpropionic acids as potent agonists of sphingosine-1-phosphate receptor-1 (S1P1) with high selectivity against all other known S1P receptor subtypes. Bioorg Med Chem Lett16:3679-83 (2006) [PubMed] Article
Yan, L; Huo, P; Doherty, G; Toth, L; Hale, JJ; Mills, SG; Hajdu, R; Keohane, CA; Rosenbach, MJ; Milligan, JA; Shei, GJ; Chrebet, G; Bergstrom, J; Card, D; Quackenbush, E; Wickham, A; Mandala, SM Discovery of 3-arylpropionic acids as potent agonists of sphingosine-1-phosphate receptor-1 (S1P1) with high selectivity against all other known S1P receptor subtypes. Bioorg Med Chem Lett16:3679-83 (2006) [PubMed] Article