| Reaction Details |
|---|
 | Report a problem with these data |
| Target | 5-hydroxytryptamine receptor 1B |
|---|
| Ligand | BDBM50199565 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_432902 (CHEMBL915028) |
|---|
| Ki | 30±n/a nM |
|---|
| Citation |  Horchler, CL; McCauley, JP; Hall, JE; Snyder, DH; Craig Moore, W; Hudzik, TJ; Chapdelaine, MJ Synthesis of novel quinolone and quinoline-2-carboxylic acid (4-morpholin-4-yl-phenyl)amides: a late-stage diversification approach to potent 5HT1B antagonists. Bioorg Med Chem15:939-50 (2006) [PubMed] Article Horchler, CL; McCauley, JP; Hall, JE; Snyder, DH; Craig Moore, W; Hudzik, TJ; Chapdelaine, MJ Synthesis of novel quinolone and quinoline-2-carboxylic acid (4-morpholin-4-yl-phenyl)amides: a late-stage diversification approach to potent 5HT1B antagonists. Bioorg Med Chem15:939-50 (2006) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| 5-hydroxytryptamine receptor 1B |
|---|
| Name: | 5-hydroxytryptamine receptor 1B |
|---|
| Synonyms: | 5-HT-1B | 5-HT-1D-beta | 5-HT1B | 5-hydroxytryptamine receptor 1B (5-HT1B) | 5HT1B_HUMAN | HTR1B | HTR1DB | S12 | Serotonin (5-HT) receptor | Serotonin 1D beta receptor | Serotonin Receptor 1B |
|---|
| Type: | G Protein-Coupled Receptor (GPCR) |
|---|
| Mol. Mass.: | 43579.17 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | Receptor binding assays were performed using human clone stably expressed in CHO cells |
|---|
| Residue: | 390 |
|---|
| Sequence: | MEEPGAQCAPPPPAGSETWVPQANLSSAPSQNCSAKDYIYQDSISLPWKVLLVMLLALIT
LATTLSNAFVIATVYRTRKLHTPANYLIASLAVTDLLVSILVMPISTMYTVTGRWTLGQV
VCDFWLSSDITCCTASILHLCVIALDRYWAITDAVEYSAKRTPKRAAVMIALVWVFSISI
SLPPFFWRQAKAEEEVSECVVNTDHILYTVYSTVGAFYFPTLLLIALYGRIYVEARSRIL
KQTPNRTGKRLTRAQLITDSPGSTSSVTSINSRVPDVPSESGSPVYVNQVKVRVSDALLE
KKKLMAARERKATKTLGIILGAFIVCWLPFFIISLVMPICKDACWFHLAIFDFFTWLGYL
NSLINPIIYTMSNEDFKQAFHKLIRFKCTS
|
|
|
|---|
| BDBM50199565 |
|---|
| n/a |
|---|
| Name | BDBM50199565 |
|---|
| Synonyms: | 6-fluoro-4-methoxy-8-(4-methyl-piperazin-1-yl)-quinoline-2-carboxylic acid (4-morpholin-4-yl-phenyl)amide | CHEMBL224267 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C26H30FN5O3 |
|---|
| Mol. Mass. | 479.5465 |
|---|
| SMILES | COc1cc(nc2c(cc(F)cc12)N1CCN(C)CC1)C(=O)Nc1ccc(cc1)N1CCOCC1 |
|---|
| Structure | 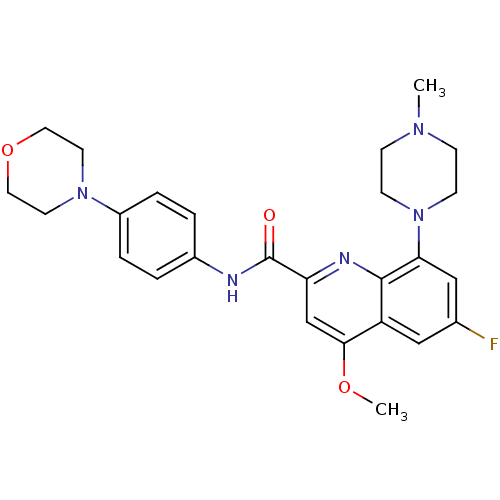
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Horchler, CL; McCauley, JP; Hall, JE; Snyder, DH; Craig Moore, W; Hudzik, TJ; Chapdelaine, MJ Synthesis of novel quinolone and quinoline-2-carboxylic acid (4-morpholin-4-yl-phenyl)amides: a late-stage diversification approach to potent 5HT1B antagonists. Bioorg Med Chem15:939-50 (2006) [PubMed] Article
Horchler, CL; McCauley, JP; Hall, JE; Snyder, DH; Craig Moore, W; Hudzik, TJ; Chapdelaine, MJ Synthesis of novel quinolone and quinoline-2-carboxylic acid (4-morpholin-4-yl-phenyl)amides: a late-stage diversification approach to potent 5HT1B antagonists. Bioorg Med Chem15:939-50 (2006) [PubMed] Article