| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Prolyl endopeptidase |
|---|
| Ligand | BDBM50200723 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_422119 (CHEMBL908233) |
|---|
| Ki | 2.8±n/a nM |
|---|
| Citation |  Tran, T; Quan, C; Edosada, CY; Mayeda, M; Wiesmann, C; Sutherlin, D; Wolf, BB Synthesis and structure-activity relationship of N-acyl-Gly-, N-acyl-Sar- and N-blocked-boroPro inhibitors of FAP, DPP4, and POP. Bioorg Med Chem Lett17:1438-42 (2007) [PubMed] Article Tran, T; Quan, C; Edosada, CY; Mayeda, M; Wiesmann, C; Sutherlin, D; Wolf, BB Synthesis and structure-activity relationship of N-acyl-Gly-, N-acyl-Sar- and N-blocked-boroPro inhibitors of FAP, DPP4, and POP. Bioorg Med Chem Lett17:1438-42 (2007) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Prolyl endopeptidase |
|---|
| Name: | Prolyl endopeptidase |
|---|
| Synonyms: | PE | PEP | POP | PPCE_HUMAN | PREP | Post-proline cleaving enzyme | Prolyl oligopeptidase |
|---|
| Type: | Enzyme |
|---|
| Mol. Mass.: | 80688.50 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | P48147 |
|---|
| Residue: | 710 |
|---|
| Sequence: | MLSLQYPDVYRDETAVQDYHGHKICDPYAWLEDPDSEQTKAFVEAQNKITVPFLEQCPIR
GLYKERMTELYDYPKYSCHFKKGKRYFYFYNTGLQNQRVLYVQDSLEGEARVFLDPNILS
DDGTVALRGYAFSEDGEYFAYGLSASGSDWVTIKFMKVDGAKELPDVLERVKFSCMAWTH
DGKGMFYNSYPQQDGKSDGTETSTNLHQKLYYHVLGTDQSEDILCAEFPDEPKWMGGAEL
SDDGRYVLLSIREGCDPVNRLWYCDLQQESSGIAGILKWVKLIDNFEGEYDYVTNEGTVF
TFKTNRQSPNYRVINIDFRDPEESKWKVLVPEHEKDVLEWIACVRSNFLVLCYLHDVKNI
LQLHDLTTGALLKTFPLDVGSIVGYSGQKKDTEIFYQFTSFLSPGIIYHCDLTKEELEPR
VFREVTVKGIDASDYQTVQIFYPSKDGTKIPMFIVHKKGIKLDGSHPAFLYGYGGFNISI
TPNYSVSRLIFVRHMGGILAVANIRGGGEYGETWHKGGILANKQNCFDDFQCAAEYLIKE
GYTSPKRLTINGGSNGGLLVAACANQRPDLFGCVIAQVGVMDMLKFHKYTIGHAWTTDYG
CSDSKQHFEWLVKYSPLHNVKLPEADDIQYPSMLLLTADHDDRVVPLHSLKFIATLQYIV
GRSRKQSNPLLIHVDTKAGHGAGKPTAKVIEEVSDMFAFIARCLNVDWIP
|
|
|
|---|
| BDBM50200723 |
|---|
| n/a |
|---|
| Name | BDBM50200723 |
|---|
| Synonyms: | (R)-1-(2-(1-oxoisoindolin-2-yl)acetyl)pyrrolidin-2-ylboronic acid | CHEMBL387050 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C14H17BN2O4 |
|---|
| Mol. Mass. | 288.107 |
|---|
| SMILES | OB(O)[C@@H]1CCCN1C(=O)CN1Cc2ccccc2C1=O |
|---|
| Structure | 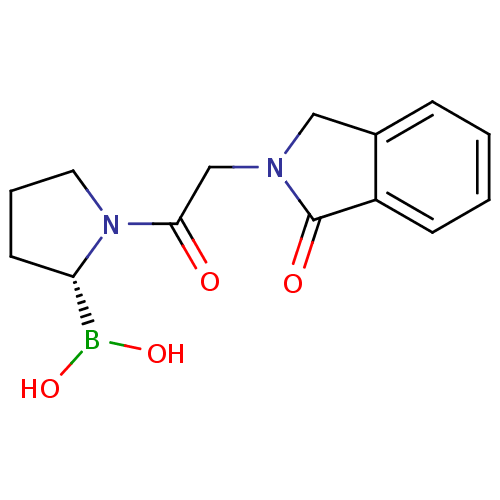
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Tran, T; Quan, C; Edosada, CY; Mayeda, M; Wiesmann, C; Sutherlin, D; Wolf, BB Synthesis and structure-activity relationship of N-acyl-Gly-, N-acyl-Sar- and N-blocked-boroPro inhibitors of FAP, DPP4, and POP. Bioorg Med Chem Lett17:1438-42 (2007) [PubMed] Article
Tran, T; Quan, C; Edosada, CY; Mayeda, M; Wiesmann, C; Sutherlin, D; Wolf, BB Synthesis and structure-activity relationship of N-acyl-Gly-, N-acyl-Sar- and N-blocked-boroPro inhibitors of FAP, DPP4, and POP. Bioorg Med Chem Lett17:1438-42 (2007) [PubMed] Article