| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Receptor tyrosine-protein kinase erbB-2 |
|---|
| Ligand | BDBM50203526 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_425536 (CHEMBL911373) |
|---|
| IC50 | 18±n/a nM |
|---|
| Citation |  Yao, W; Zhuo, J; Burns, DM; Xu, M; Zhang, C; Li, YL; Qian, DQ; He, C; Weng, L; Shi, E; Lin, Q; Agrios, C; Burn, TC; Caulder, E; Covington, MB; Fridman, JS; Friedman, S; Katiyar, K; Hollis, G; Li, Y; Liu, C; Liu, X; Marando, CA; Newton, R; Pan, M; Scherle, P; Taylor, N; Vaddi, K; Wasserman, ZR; Wynn, R; Yeleswaram, S; Jalluri, R; Bower, M; Zhou, BB; Metcalf, B Discovery of a potent, selective, and orally active human epidermal growth factor receptor-2 sheddase inhibitor for the treatment of cancer. J Med Chem50:603-6 (2007) [PubMed] Article Yao, W; Zhuo, J; Burns, DM; Xu, M; Zhang, C; Li, YL; Qian, DQ; He, C; Weng, L; Shi, E; Lin, Q; Agrios, C; Burn, TC; Caulder, E; Covington, MB; Fridman, JS; Friedman, S; Katiyar, K; Hollis, G; Li, Y; Liu, C; Liu, X; Marando, CA; Newton, R; Pan, M; Scherle, P; Taylor, N; Vaddi, K; Wasserman, ZR; Wynn, R; Yeleswaram, S; Jalluri, R; Bower, M; Zhou, BB; Metcalf, B Discovery of a potent, selective, and orally active human epidermal growth factor receptor-2 sheddase inhibitor for the treatment of cancer. J Med Chem50:603-6 (2007) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Receptor tyrosine-protein kinase erbB-2 |
|---|
| Name: | Receptor tyrosine-protein kinase erbB-2 |
|---|
| Synonyms: | 2.7.10.1 | C-erbB-2 | CD_antigen=CD340 | ERBB2 | ERBB2_HUMAN | ErbB-2/ErbB-3 heterodimer | FASN/HER2 | HER-2 Substrate | HER2 | MLN 19 | MLN19 | Metastatic lymph node gene 19 protein | NEU | NGL | Proto-oncogene Neu | Proto-oncogene c-ErbB-2 | Tyrosine kinase-type cell surface receptor HER2 | p185erbB2 |
|---|
| Type: | n/a |
|---|
| Mol. Mass.: | 137894.47 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | P04626 |
|---|
| Residue: | 1255 |
|---|
| Sequence: | MELAALCRWGLLLALLPPGAASTQVCTGTDMKLRLPASPETHLDMLRHLYQGCQVVQGNL
ELTYLPTNASLSFLQDIQEVQGYVLIAHNQVRQVPLQRLRIVRGTQLFEDNYALAVLDNG
DPLNNTTPVTGASPGGLRELQLRSLTEILKGGVLIQRNPQLCYQDTILWKDIFHKNNQLA
LTLIDTNRSRACHPCSPMCKGSRCWGESSEDCQSLTRTVCAGGCARCKGPLPTDCCHEQC
AAGCTGPKHSDCLACLHFNHSGICELHCPALVTYNTDTFESMPNPEGRYTFGASCVTACP
YNYLSTDVGSCTLVCPLHNQEVTAEDGTQRCEKCSKPCARVCYGLGMEHLREVRAVTSAN
IQEFAGCKKIFGSLAFLPESFDGDPASNTAPLQPEQLQVFETLEEITGYLYISAWPDSLP
DLSVFQNLQVIRGRILHNGAYSLTLQGLGISWLGLRSLRELGSGLALIHHNTHLCFVHTV
PWDQLFRNPHQALLHTANRPEDECVGEGLACHQLCARGHCWGPGPTQCVNCSQFLRGQEC
VEECRVLQGLPREYVNARHCLPCHPECQPQNGSVTCFGPEADQCVACAHYKDPPFCVARC
PSGVKPDLSYMPIWKFPDEEGACQPCPINCTHSCVDLDDKGCPAEQRASPLTSIISAVVG
ILLVVVLGVVFGILIKRRQQKIRKYTMRRLLQETELVEPLTPSGAMPNQAQMRILKETEL
RKVKVLGSGAFGTVYKGIWIPDGENVKIPVAIKVLRENTSPKANKEILDEAYVMAGVGSP
YVSRLLGICLTSTVQLVTQLMPYGCLLDHVRENRGRLGSQDLLNWCMQIAKGMSYLEDVR
LVHRDLAARNVLVKSPNHVKITDFGLARLLDIDETEYHADGGKVPIKWMALESILRRRFT
HQSDVWSYGVTVWELMTFGAKPYDGIPAREIPDLLEKGERLPQPPICTIDVYMIMVKCWM
IDSECRPRFRELVSEFSRMARDPQRFVVIQNEDLGPASPLDSTFYRSLLEDDDMGDLVDA
EEYLVPQQGFFCPDPAPGAGGMVHHRHRSSSTRSGGGDLTLGLEPSEEEAPRSPLAPSEG
AGSDVFDGDLGMGAAKGLQSLPTHDPSPLQRYSEDPTVPLPSETDGYVAPLTCSPQPEYV
NQPDVRPQPPSPREGPLPAARPAGATLERPKTLSPGKNGVVKDVFAFGGAVENPEYLTPQ
GGAAPQPHPPPAFSPAFDNLYYWDQDPPERGAPPSTFKGTPTAENPEYLGLDVPV
|
|
|
|---|
| BDBM50203526 |
|---|
| n/a |
|---|
| Name | BDBM50203526 |
|---|
| Synonyms: | (6S,7S)-6-(4-phenyl-3,6-dihydro-2H-pyridine-1-carbonyl)-5-aza-spiro[2.5]octane-7-carboxylic acid hydroxyamide | (6S,7S)-N-hydroxy-6-(4-phenyl-1,2,3,6-tetrahydropyridine-1-carbonyl)-5-azaspiro[2.5]octane-7-carboxamide | (6S,7S)-N-hydroxy-6-[(4-phenyl-3,6-dihydropyridin-1(2H)-yl)carbonyl]-5-azaspiro[2.5]octane-7-carboxamide | CHEMBL373532 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C20H25N3O3 |
|---|
| Mol. Mass. | 355.4308 |
|---|
| SMILES | ONC(=O)[C@H]1CC2(CC2)CN[C@@H]1C(=O)N1CCC(=CC1)c1ccccc1 |c:19| |
|---|
| Structure | 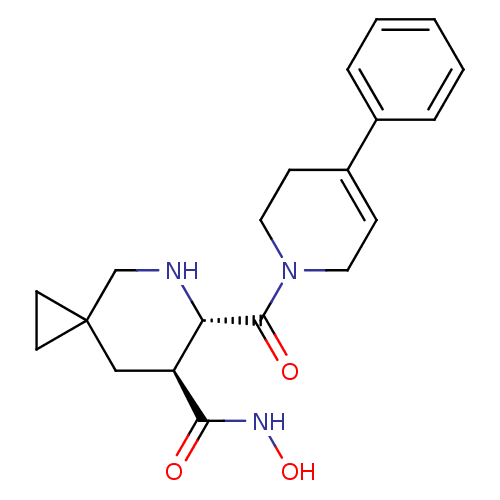
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Yao, W; Zhuo, J; Burns, DM; Xu, M; Zhang, C; Li, YL; Qian, DQ; He, C; Weng, L; Shi, E; Lin, Q; Agrios, C; Burn, TC; Caulder, E; Covington, MB; Fridman, JS; Friedman, S; Katiyar, K; Hollis, G; Li, Y; Liu, C; Liu, X; Marando, CA; Newton, R; Pan, M; Scherle, P; Taylor, N; Vaddi, K; Wasserman, ZR; Wynn, R; Yeleswaram, S; Jalluri, R; Bower, M; Zhou, BB; Metcalf, B Discovery of a potent, selective, and orally active human epidermal growth factor receptor-2 sheddase inhibitor for the treatment of cancer. J Med Chem50:603-6 (2007) [PubMed] Article
Yao, W; Zhuo, J; Burns, DM; Xu, M; Zhang, C; Li, YL; Qian, DQ; He, C; Weng, L; Shi, E; Lin, Q; Agrios, C; Burn, TC; Caulder, E; Covington, MB; Fridman, JS; Friedman, S; Katiyar, K; Hollis, G; Li, Y; Liu, C; Liu, X; Marando, CA; Newton, R; Pan, M; Scherle, P; Taylor, N; Vaddi, K; Wasserman, ZR; Wynn, R; Yeleswaram, S; Jalluri, R; Bower, M; Zhou, BB; Metcalf, B Discovery of a potent, selective, and orally active human epidermal growth factor receptor-2 sheddase inhibitor for the treatment of cancer. J Med Chem50:603-6 (2007) [PubMed] Article