| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Prostaglandin D2 receptor |
|---|
| Ligand | BDBM50205274 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_425870 (CHEMBL856874) |
|---|
| Ki | 1.5±n/a nM |
|---|
| Citation |  Sturino, CF; O'Neill, G; Lachance, N; Boyd, M; Berthelette, C; Labelle, M; Li, L; Roy, B; Scheigetz, J; Tsou, N; Aubin, Y; Bateman, KP; Chauret, N; Day, SH; Lévesque, JF; Seto, C; Silva, JH; Trimble, LA; Carriere, MC; Denis, D; Greig, G; Kargman, S; Lamontagne, S; Mathieu, MC; Sawyer, N; Slipetz, D; Abraham, WM; Jones, T; McAuliffe, M; Piechuta, H; Nicoll-Griffith, DA; Wang, Z; Zamboni, R; Young, RN; Metters, KM Discovery of a potent and selective prostaglandin D2 receptor antagonist, [(3R)-4-(4-chloro-benzyl)-7-fluoro-5-(methylsulfonyl)-1,2,3,4-tetrahydrocyclopenta[b]indol-3-yl]-acetic acid (MK-0524). J Med Chem50:794-806 (2007) [PubMed] Article Sturino, CF; O'Neill, G; Lachance, N; Boyd, M; Berthelette, C; Labelle, M; Li, L; Roy, B; Scheigetz, J; Tsou, N; Aubin, Y; Bateman, KP; Chauret, N; Day, SH; Lévesque, JF; Seto, C; Silva, JH; Trimble, LA; Carriere, MC; Denis, D; Greig, G; Kargman, S; Lamontagne, S; Mathieu, MC; Sawyer, N; Slipetz, D; Abraham, WM; Jones, T; McAuliffe, M; Piechuta, H; Nicoll-Griffith, DA; Wang, Z; Zamboni, R; Young, RN; Metters, KM Discovery of a potent and selective prostaglandin D2 receptor antagonist, [(3R)-4-(4-chloro-benzyl)-7-fluoro-5-(methylsulfonyl)-1,2,3,4-tetrahydrocyclopenta[b]indol-3-yl]-acetic acid (MK-0524). J Med Chem50:794-806 (2007) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Prostaglandin D2 receptor |
|---|
| Name: | Prostaglandin D2 receptor |
|---|
| Synonyms: | PD2R_HUMAN | PTGDR | Prostaglandin D2 | Prostaglandin D2 receptor | Prostanoid DP receptor |
|---|
| Type: | Enzyme |
|---|
| Mol. Mass.: | 40288.87 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | Q13258 |
|---|
| Residue: | 359 |
|---|
| Sequence: | MKSPFYRCQNTTSVEKGNSAVMGGVLFSTGLLGNLLALGLLARSGLGWCSRRPLRPLPSV
FYMLVCGLTVTDLLGKCLLSPVVLAAYAQNRSLRVLAPALDNSLCQAFAFFMSFFGLSST
LQLLAMALECWLSLGHPFFYRRHITLRLGALVAPVVSAFSLAFCALPFMGFGKFVQYCPG
TWCFIQMVHEEGSLSVLGYSVLYSSLMALLVLATVLCNLGAMRNLYAMHRRLQRHPRSCT
RDCAEPRADGREASPQPLEELDHLLLLALMTVLFTMCSLPVIYRAYYGAFKDVKEKNRTS
EEAEDLRALRFLSVISIVDPWIFIIFRSPVFRIFFHKIFIRPLRYRSRCSNSTNMESSL
|
|
|
|---|
| BDBM50205274 |
|---|
| n/a |
|---|
| Name | BDBM50205274 |
|---|
| Synonyms: | CHEMBL426387 | [(3R)-5-bromo-4-(4-chlorobenzyl)-7-fluoro-1,2,3,4-tetrahydrocyclopenta[b]indol-3-yl]acetic acid |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C20H16BrClFNO2 |
|---|
| Mol. Mass. | 436.702 |
|---|
| SMILES | OC(=O)C[C@H]1CCc2c1n(Cc1ccc(Cl)cc1)c1c(Br)cc(F)cc21 |
|---|
| Structure | 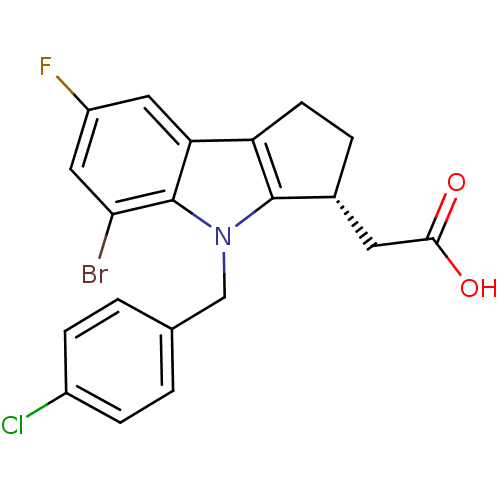
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Sturino, CF; O'Neill, G; Lachance, N; Boyd, M; Berthelette, C; Labelle, M; Li, L; Roy, B; Scheigetz, J; Tsou, N; Aubin, Y; Bateman, KP; Chauret, N; Day, SH; Lévesque, JF; Seto, C; Silva, JH; Trimble, LA; Carriere, MC; Denis, D; Greig, G; Kargman, S; Lamontagne, S; Mathieu, MC; Sawyer, N; Slipetz, D; Abraham, WM; Jones, T; McAuliffe, M; Piechuta, H; Nicoll-Griffith, DA; Wang, Z; Zamboni, R; Young, RN; Metters, KM Discovery of a potent and selective prostaglandin D2 receptor antagonist, [(3R)-4-(4-chloro-benzyl)-7-fluoro-5-(methylsulfonyl)-1,2,3,4-tetrahydrocyclopenta[b]indol-3-yl]-acetic acid (MK-0524). J Med Chem50:794-806 (2007) [PubMed] Article
Sturino, CF; O'Neill, G; Lachance, N; Boyd, M; Berthelette, C; Labelle, M; Li, L; Roy, B; Scheigetz, J; Tsou, N; Aubin, Y; Bateman, KP; Chauret, N; Day, SH; Lévesque, JF; Seto, C; Silva, JH; Trimble, LA; Carriere, MC; Denis, D; Greig, G; Kargman, S; Lamontagne, S; Mathieu, MC; Sawyer, N; Slipetz, D; Abraham, WM; Jones, T; McAuliffe, M; Piechuta, H; Nicoll-Griffith, DA; Wang, Z; Zamboni, R; Young, RN; Metters, KM Discovery of a potent and selective prostaglandin D2 receptor antagonist, [(3R)-4-(4-chloro-benzyl)-7-fluoro-5-(methylsulfonyl)-1,2,3,4-tetrahydrocyclopenta[b]indol-3-yl]-acetic acid (MK-0524). J Med Chem50:794-806 (2007) [PubMed] Article