| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Cytochrome P450 2D6 |
|---|
| Ligand | BDBM50210019 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_458976 (CHEMBL925068) |
|---|
| IC50 | 410±n/a nM |
|---|
| Citation |  Pruitt, JR; Batt, DG; Wacker, DA; Bostrom, LL; Booker, SK; McLaughlin, E; Houghton, GC; Varnes, JG; Christ, DD; Covington, M; Das, AM; Davies, P; Graden, D; Kariv, I; Orlovsky, Y; Stowell, NC; Vaddi, KG; Wadman, EA; Welch, PK; Yeleswaram, S; Solomon, KA; Newton, RC; Decicco, CP; Carter, PH; Ko, SS CC chemokine receptor-3 (CCR3) antagonists: improving the selectivity of DPC168 by reducing central ring lipophilicity. Bioorg Med Chem Lett17:2992-7 (2007) [PubMed] Article Pruitt, JR; Batt, DG; Wacker, DA; Bostrom, LL; Booker, SK; McLaughlin, E; Houghton, GC; Varnes, JG; Christ, DD; Covington, M; Das, AM; Davies, P; Graden, D; Kariv, I; Orlovsky, Y; Stowell, NC; Vaddi, KG; Wadman, EA; Welch, PK; Yeleswaram, S; Solomon, KA; Newton, RC; Decicco, CP; Carter, PH; Ko, SS CC chemokine receptor-3 (CCR3) antagonists: improving the selectivity of DPC168 by reducing central ring lipophilicity. Bioorg Med Chem Lett17:2992-7 (2007) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Cytochrome P450 2D6 |
|---|
| Name: | Cytochrome P450 2D6 |
|---|
| Synonyms: | CP2D6_HUMAN | CYP2D6 | CYP2DL1 | CYPIID6 | Cytochrome P450 2D6 (CYP2D6) | Debrisoquine 4-hydroxylase | P450-DB1 |
|---|
| Type: | Protein |
|---|
| Mol. Mass.: | 55774.82 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | P10635 |
|---|
| Residue: | 497 |
|---|
| Sequence: | MGLEALVPLAVIVAIFLLLVDLMHRRQRWAARYPPGPLPLPGLGNLLHVDFQNTPYCFDQ
LRRRFGDVFSLQLAWTPVVVLNGLAAVREALVTHGEDTADRPPVPITQILGFGPRSQGVF
LARYGPAWREQRRFSVSTLRNLGLGKKSLEQWVTEEAACLCAAFANHSGRPFRPNGLLDK
AVSNVIASLTCGRRFEYDDPRFLRLLDLAQEGLKEESGFLREVLNAVPVLLHIPALAGKV
LRFQKAFLTQLDELLTEHRMTWDPAQPPRDLTEAFLAEMEKAKGNPESSFNDENLRIVVA
DLFSAGMVTTSTTLAWGLLLMILHPDVQRRVQQEIDDVIGQVRRPEMGDQAHMPYTTAVI
HEVQRFGDIVPLGVTHMTSRDIEVQGFRIPKGTTLITNLSSVLKDEAVWEKPFRFHPEHF
LDAQGHFVKPEAFLPFSAGRRACLGEPLARMELFLFFTSLLQHFSFSVPTGQPRPSHHGV
FAFLVSPSPYELCAVPR
|
|
|
|---|
| BDBM50210019 |
|---|
| n/a |
|---|
| Name | BDBM50210019 |
|---|
| Synonyms: | 1-((3S,4S)-4-(((S)-3-(4-fluorobenzyl)piperidin-1-yl)methyl)-1-(cyanomethyl)piperidin-3-yl)-3-(5-acetyl-4-methylthiazol-2-yl)urea | CHEMBL404857 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C27H35FN6O2S |
|---|
| Mol. Mass. | 526.669 |
|---|
| SMILES | CC(=O)c1sc(NC(=O)N[C@@H]2CN(CC#N)CC[C@H]2CN2CCC[C@@H](Cc3ccc(F)cc3)C2)nc1C |
|---|
| Structure | 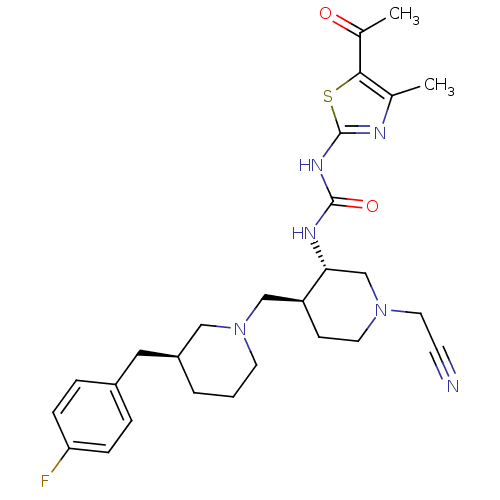
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Pruitt, JR; Batt, DG; Wacker, DA; Bostrom, LL; Booker, SK; McLaughlin, E; Houghton, GC; Varnes, JG; Christ, DD; Covington, M; Das, AM; Davies, P; Graden, D; Kariv, I; Orlovsky, Y; Stowell, NC; Vaddi, KG; Wadman, EA; Welch, PK; Yeleswaram, S; Solomon, KA; Newton, RC; Decicco, CP; Carter, PH; Ko, SS CC chemokine receptor-3 (CCR3) antagonists: improving the selectivity of DPC168 by reducing central ring lipophilicity. Bioorg Med Chem Lett17:2992-7 (2007) [PubMed] Article
Pruitt, JR; Batt, DG; Wacker, DA; Bostrom, LL; Booker, SK; McLaughlin, E; Houghton, GC; Varnes, JG; Christ, DD; Covington, M; Das, AM; Davies, P; Graden, D; Kariv, I; Orlovsky, Y; Stowell, NC; Vaddi, KG; Wadman, EA; Welch, PK; Yeleswaram, S; Solomon, KA; Newton, RC; Decicco, CP; Carter, PH; Ko, SS CC chemokine receptor-3 (CCR3) antagonists: improving the selectivity of DPC168 by reducing central ring lipophilicity. Bioorg Med Chem Lett17:2992-7 (2007) [PubMed] Article