| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Glyoxalase I |
|---|
| Ligand | BDBM50213420 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_456126 (CHEMBL888135) |
|---|
| Ki | 66880±n/a nM |
|---|
| Citation |  More, SS; Vince, R Design, synthesis, and binding studies of bidentate Zn-chelating peptidic inhibitors of glyoxalase-I. Bioorg Med Chem Lett17:3793-7 (2007) [PubMed] Article More, SS; Vince, R Design, synthesis, and binding studies of bidentate Zn-chelating peptidic inhibitors of glyoxalase-I. Bioorg Med Chem Lett17:3793-7 (2007) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Glyoxalase I |
|---|
| Name: | Glyoxalase I |
|---|
| Synonyms: | GLO1 | LGUL_YEAST | Lactoylglutathione lyase |
|---|
| Type: | PROTEIN |
|---|
| Mol. Mass.: | 37210.98 |
|---|
| Organism: | Saccharomyces cerevisiae |
|---|
| Description: | ChEMBL_571116 |
|---|
| Residue: | 326 |
|---|
| Sequence: | MSTDSTRYPIQIEKASNDPTLLLNHTCLRVKDPARTVKFYTEHFGMKLLSRKDFEEAKFS
LYFLSFPKDDIPKNKNGEPDVFSAHGVLELTHNWGTEKNPDYKINNGNEEPHRGFGHICF
SVSDINKTCEELESQGVKFKKRLSEGRQKDIAFALGPDGYWIELITYSREGQEYPKGSVG
NKFNHTMIRIKNPTRSLEFYQNVLGMKLLRTSEHESAKFTLYFLGYGVPKTDSVFSCESV
LELTHNWGTENDPNFHYHNGNSEPQGYGHICISCDDAGALCKEIEVKYGDKIQWSPKFNQ
GRMKNIAFLKDPDGYSIEVVPHGLIA
|
|
|
|---|
| BDBM50213420 |
|---|
| n/a |
|---|
| Name | BDBM50213420 |
|---|
| Synonyms: | (S)-5-((S)-7-(4-bromobenzyloxy)-1-(carboxymethylamino)-1,5,7-trioxoheptan-2-ylamino)-2-amino-5-oxopentanoic acid | CHEMBL246554 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C21H26BrN3O9 |
|---|
| Mol. Mass. | 544.35 |
|---|
| SMILES | N[C@@H](CCC(=O)N[C@@H](CCC(=O)CC(=O)OCc1ccc(Br)cc1)C(=O)NCC(O)=O)C(O)=O |
|---|
| Structure | 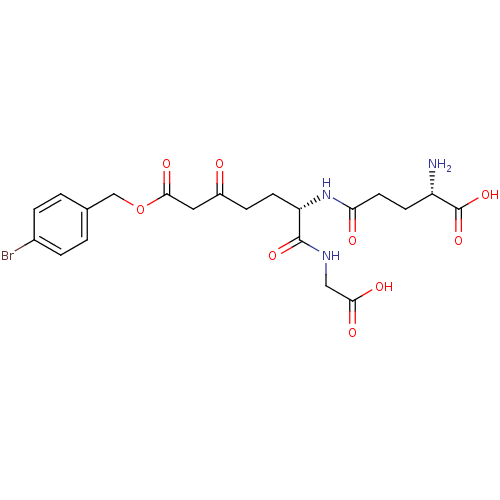
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 More, SS; Vince, R Design, synthesis, and binding studies of bidentate Zn-chelating peptidic inhibitors of glyoxalase-I. Bioorg Med Chem Lett17:3793-7 (2007) [PubMed] Article
More, SS; Vince, R Design, synthesis, and binding studies of bidentate Zn-chelating peptidic inhibitors of glyoxalase-I. Bioorg Med Chem Lett17:3793-7 (2007) [PubMed] Article