| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Plasminogen |
|---|
| Ligand | BDBM50218290 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_448746 (CHEMBL897893) |
|---|
| Ki | >22000±n/a nM |
|---|
| Citation |  Qiao, JX; Wang, TC; Wang, GZ; Cheney, DL; He, K; Rendina, AR; Xin, B; Luettgen, JM; Knabb, RM; Wexler, RR; Lam, PY Enantiopure five-membered cyclicdiamine derivatives as potent and selective inhibitors of factor Xa. Improving in vitro metabolic stability via core modifications. Bioorg Med Chem Lett17:5041-8 (2007) [PubMed] Article Qiao, JX; Wang, TC; Wang, GZ; Cheney, DL; He, K; Rendina, AR; Xin, B; Luettgen, JM; Knabb, RM; Wexler, RR; Lam, PY Enantiopure five-membered cyclicdiamine derivatives as potent and selective inhibitors of factor Xa. Improving in vitro metabolic stability via core modifications. Bioorg Med Chem Lett17:5041-8 (2007) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Plasminogen |
|---|
| Name: | Plasminogen |
|---|
| Synonyms: | Activation peptide | Angiostatin | PLG | PLMN_HUMAN | Plasmin | Plasmin heavy chain A | Plasmin heavy chain A, short form | Plasmin light chain B |
|---|
| Type: | Enzyme |
|---|
| Mol. Mass.: | 90579.18 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | n/a |
|---|
| Residue: | 810 |
|---|
| Sequence: | MEHKEVVLLLLLFLKSGQGEPLDDYVNTQGASLFSVTKKQLGAGSIEECAAKCEEDEEFT
CRAFQYHSKEQQCVIMAENRKSSIIIRMRDVVLFEKKVYLSECKTGNGKNYRGTMSKTKN
GITCQKWSSTSPHRPRFSPATHPSEGLEENYCRNPDNDPQGPWCYTTDPEKRYDYCDILE
CEEECMHCSGENYDGKISKTMSGLECQAWDSQSPHAHGYIPSKFPNKNLKKNYCRNPDRE
LRPWCFTTDPNKRWELCDIPRCTTPPPSSGPTYQCLKGTGENYRGNVAVTVSGHTCQHWS
AQTPHTHNRTPENFPCKNLDENYCRNPDGKRAPWCHTTNSQVRWEYCKIPSCDSSPVSTE
QLAPTAPPELTPVVQDCYHGDGQSYRGTSSTTTTGKKCQSWSSMTPHRHQKTPENYPNAG
LTMNYCRNPDADKGPWCFTTDPSVRWEYCNLKKCSGTEASVVAPPPVVLLPDVETPSEED
CMFGNGKGYRGKRATTVTGTPCQDWAAQEPHRHSIFTPETNPRAGLEKNYCRNPDGDVGG
PWCYTTNPRKLYDYCDVPQCAAPSFDCGKPQVEPKKCPGRVVGGCVAHPHSWPWQVSLRT
RFGMHFCGGTLISPEWVLTAAHCLEKSPRPSSYKVILGAHQEVNLEPHVQEIEVSRLFLE
PTRKDIALLKLSSPAVITDKVIPACLPSPNYVVADRTECFITGWGETQGTFGAGLLKEAQ
LPVIENKVCNRYEFLNGRVQSTELCAGHLAGGTDSCQGDSGGPLVCFEKDKYILQGVTSW
GLGCARPNKPGVYVRVSRFVTWIEGVMRNN
|
|
|
|---|
| BDBM50218290 |
|---|
| n/a |
|---|
| Name | BDBM50218290 |
|---|
| Synonyms: | 5-chloro-N-((1R,2S,4S)-4-(hydroxymethyl)-2-(4-(2-oxopyridin-1(2H)-yl)benzamido)cyclopentyl)thiophene-2-carboxamide | CHEMBL237065 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C23H22ClN3O4S |
|---|
| Mol. Mass. | 471.956 |
|---|
| SMILES | OC[C@@H]1C[C@@H](NC(=O)c2ccc(Cl)s2)[C@H](C1)NC(=O)c1ccc(cc1)-n1ccccc1=O |
|---|
| Structure | 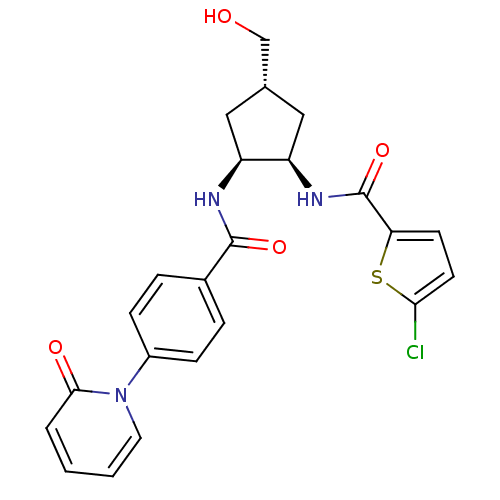
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Qiao, JX; Wang, TC; Wang, GZ; Cheney, DL; He, K; Rendina, AR; Xin, B; Luettgen, JM; Knabb, RM; Wexler, RR; Lam, PY Enantiopure five-membered cyclicdiamine derivatives as potent and selective inhibitors of factor Xa. Improving in vitro metabolic stability via core modifications. Bioorg Med Chem Lett17:5041-8 (2007) [PubMed] Article
Qiao, JX; Wang, TC; Wang, GZ; Cheney, DL; He, K; Rendina, AR; Xin, B; Luettgen, JM; Knabb, RM; Wexler, RR; Lam, PY Enantiopure five-membered cyclicdiamine derivatives as potent and selective inhibitors of factor Xa. Improving in vitro metabolic stability via core modifications. Bioorg Med Chem Lett17:5041-8 (2007) [PubMed] Article