| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Histamine H3 receptor |
|---|
| Ligand | BDBM50224191 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_450586 (CHEMBL900873) |
|---|
| Ki | 0.19±n/a nM |
|---|
| Citation |  Altenbach, RJ; Liu, H; Banfor, PN; Browman, KE; Fox, GB; Fryer, RM; Komater, VA; Krueger, KM; Marsh, K; Miller, TR; Pan, JB; Pan, L; Sun, M; Thiffault, C; Wetter, J; Zhao, C; Zhou, D; Esbenshade, TA; Hancock, AA; Cowart, MD Synthesis, potency, and in vivo profiles of quinoline containing histamine H3 receptor inverse agonists. J Med Chem50:5439-48 (2007) [PubMed] Article Altenbach, RJ; Liu, H; Banfor, PN; Browman, KE; Fox, GB; Fryer, RM; Komater, VA; Krueger, KM; Marsh, K; Miller, TR; Pan, JB; Pan, L; Sun, M; Thiffault, C; Wetter, J; Zhao, C; Zhou, D; Esbenshade, TA; Hancock, AA; Cowart, MD Synthesis, potency, and in vivo profiles of quinoline containing histamine H3 receptor inverse agonists. J Med Chem50:5439-48 (2007) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Histamine H3 receptor |
|---|
| Name: | Histamine H3 receptor |
|---|
| Synonyms: | G-protein coupled receptor 97 | GPCR97 | HH3R | HISTAMINE H3 | HRH3 | HRH3_HUMAN | Histamine H3 receptor (H3) | Histamine H3L | Histamine receptor (H3 and H4) |
|---|
| Type: | G Protein-Coupled Receptor (GPCR) |
|---|
| Mol. Mass.: | 48691.47 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | Binding assays were using CHO cells stably expressing hH3R receptors. |
|---|
| Residue: | 445 |
|---|
| Sequence: | MERAPPDGPLNASGALAGEAAAAGGARGFSAAWTAVLAALMALLIVATVLGNALVMLAFV
ADSSLRTQNNFFLLNLAISDFLVGAFCIPLYVPYVLTGRWTFGRGLCKLWLVVDYLLCTS
SAFNIVLISYDRFLSVTRAVSYRAQQGDTRRAVRKMLLVWVLAFLLYGPAILSWEYLSGG
SSIPEGHCYAEFFYNWYFLITASTLEFFTPFLSVTFFNLSIYLNIQRRTRLRLDGAREAA
GPEPPPEAQPSPPPPPGCWGCWQKGHGEAMPLHRYGVGEAAVGAEAGEATLGGGGGGGSV
ASPTSSSGSSSRGTERPRSLKRGSKPSASSASLEKRMKMVSQSFTQRFRLSRDRKVAKSL
AVIVSIFGLCWAPYTLLMIIRAACHGHCVPDYWYETSFWLLWANSAVNPVLYPLCHHSFR
RAFTKLLCPQKLKIQPHSSLEHCWK
|
|
|
|---|
| BDBM50224191 |
|---|
| n/a |
|---|
| Name | BDBM50224191 |
|---|
| Synonyms: | (R)-2-(1-(6-ethoxypyridazin-3-yl)-5-methyl-1H-pyrazol-4-yl)-6-(2-(2-methylpyrrolidin-1-yl)ethyl)quinoline | 2-[1-(6-ethoxypyridazin-3-yl)-5-methyl-1H-pyrazol-4-yl]-6-[2-(2-(R)-methylpyrrolidin-1-yl)ethyl]quinoline | CHEMBL237394 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C26H30N6O |
|---|
| Mol. Mass. | 442.556 |
|---|
| SMILES | CCOc1ccc(nn1)-n1ncc(c1C)-c1ccc2cc(CCN3CCC[C@H]3C)ccc2n1 |
|---|
| Structure | 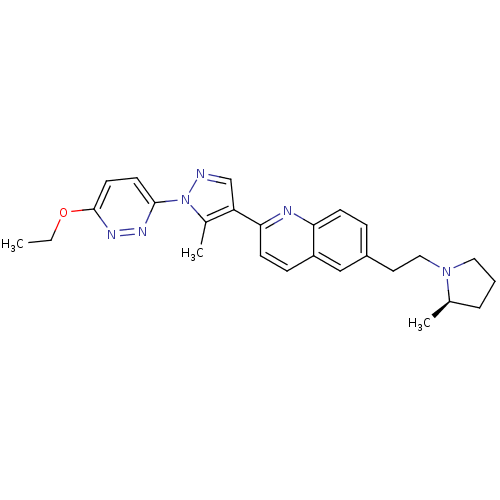
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Altenbach, RJ; Liu, H; Banfor, PN; Browman, KE; Fox, GB; Fryer, RM; Komater, VA; Krueger, KM; Marsh, K; Miller, TR; Pan, JB; Pan, L; Sun, M; Thiffault, C; Wetter, J; Zhao, C; Zhou, D; Esbenshade, TA; Hancock, AA; Cowart, MD Synthesis, potency, and in vivo profiles of quinoline containing histamine H3 receptor inverse agonists. J Med Chem50:5439-48 (2007) [PubMed] Article
Altenbach, RJ; Liu, H; Banfor, PN; Browman, KE; Fox, GB; Fryer, RM; Komater, VA; Krueger, KM; Marsh, K; Miller, TR; Pan, JB; Pan, L; Sun, M; Thiffault, C; Wetter, J; Zhao, C; Zhou, D; Esbenshade, TA; Hancock, AA; Cowart, MD Synthesis, potency, and in vivo profiles of quinoline containing histamine H3 receptor inverse agonists. J Med Chem50:5439-48 (2007) [PubMed] Article