| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Mycothiol S-conjugate amidase |
|---|
| Ligand | BDBM50227092 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_452330 (CHEMBL902567) |
|---|
| IC50 | 33000±n/a nM |
|---|
| Citation |  Metaferia, BB; Fetterolf, BJ; Shazad-Ul-Hussan, S; Moravec, M; Smith, JA; Ray, S; Gutierrez-Lugo, MT; Bewley, CA Synthesis of natural product-inspired inhibitors of Mycobacterium tuberculosis mycothiol-associated enzymes: the first inhibitors of GlcNAc-Ins deacetylase. J Med Chem50:6326-36 (2007) [PubMed] Article Metaferia, BB; Fetterolf, BJ; Shazad-Ul-Hussan, S; Moravec, M; Smith, JA; Ray, S; Gutierrez-Lugo, MT; Bewley, CA Synthesis of natural product-inspired inhibitors of Mycobacterium tuberculosis mycothiol-associated enzymes: the first inhibitors of GlcNAc-Ins deacetylase. J Med Chem50:6326-36 (2007) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Mycothiol S-conjugate amidase |
|---|
| Name: | Mycothiol S-conjugate amidase |
|---|
| Synonyms: | MCA_MYCTU | mca |
|---|
| Type: | PROTEIN |
|---|
| Mol. Mass.: | 32719.79 |
|---|
| Organism: | Mycobacterium tuberculosis |
|---|
| Description: | ChEMBL_626858 |
|---|
| Residue: | 288 |
|---|
| Sequence: | MSELRLMAVHAHPDDESSKGAATLARYADEGHRVLVVTLTGGERGEILNPAMDLPDVHGR
IAEIRRDEMTKAAEILGVEHTWLGFVDSGLPKGDLPPPLPDDCFARVPLEVSTEALVRVV
REFRPHVMTTYDENGGYPHPDHIRCHQVSVAAYEAAGDFCRFPDAGEPWTVSKLYYVHGF
LRERMQMLQDEFARHGQRGPFEQWLAYWDPDHDFLTSRVTTRVECSKYFSQRDDALRAHA
TQIDPNAEFFAAPLAWQERLWPTEEFELARSRIPARPPETELFAGIEP
|
|
|
|---|
| BDBM50227092 |
|---|
| n/a |
|---|
| Name | BDBM50227092 |
|---|
| Synonyms: | 5-(4-chlorophenyl)-N-((2R,3R,4R,5S,6R)-2-(cyclohexylthio)-tetrahydro-4,5-dihydroxy-6-(hydroxymethyl)-2H-pyran-3-yl)furan-2-carboxamide | CHEMBL238766 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C23H28ClNO6S |
|---|
| Mol. Mass. | 481.99 |
|---|
| SMILES | OC[C@@H]1O[C@@H](SC2CCCCC2)[C@@H](NC(=O)c2ccc(o2)-c2ccc(Cl)cc2)[C@H](O)[C@H]1O |
|---|
| Structure | 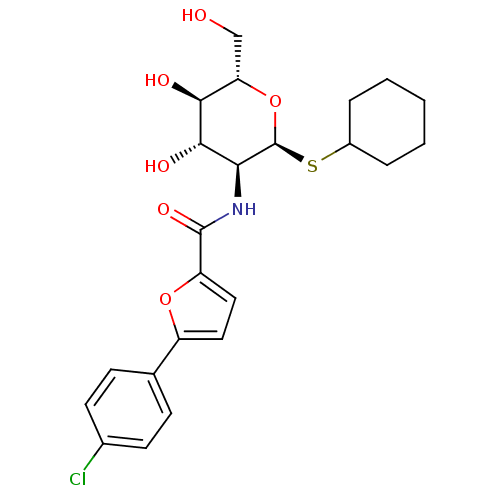
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Metaferia, BB; Fetterolf, BJ; Shazad-Ul-Hussan, S; Moravec, M; Smith, JA; Ray, S; Gutierrez-Lugo, MT; Bewley, CA Synthesis of natural product-inspired inhibitors of Mycobacterium tuberculosis mycothiol-associated enzymes: the first inhibitors of GlcNAc-Ins deacetylase. J Med Chem50:6326-36 (2007) [PubMed] Article
Metaferia, BB; Fetterolf, BJ; Shazad-Ul-Hussan, S; Moravec, M; Smith, JA; Ray, S; Gutierrez-Lugo, MT; Bewley, CA Synthesis of natural product-inspired inhibitors of Mycobacterium tuberculosis mycothiol-associated enzymes: the first inhibitors of GlcNAc-Ins deacetylase. J Med Chem50:6326-36 (2007) [PubMed] Article