| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Cathepsin E |
|---|
| Ligand | BDBM50271626 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_556483 (CHEMBL956478) |
|---|
| Ki | 30±n/a nM |
|---|
| Citation |  Cumming, JN; Le, TX; Babu, S; Carroll, C; Chen, X; Favreau, L; Gaspari, P; Guo, T; Hobbs, DW; Huang, Y; Iserloh, U; Kennedy, ME; Kuvelkar, R; Li, G; Lowrie, J; McHugh, NA; Ozgur, L; Pan, J; Parker, EM; Saionz, K; Stamford, AW; Strickland, C; Tadesse, D; Voigt, J; Wang, L; Wu, Y; Zhang, L; Zhang, Q Rational design of novel, potent piperazinone and imidazolidinone BACE1 inhibitors. Bioorg Med Chem Lett18:3236-41 (2008) [PubMed] Article Cumming, JN; Le, TX; Babu, S; Carroll, C; Chen, X; Favreau, L; Gaspari, P; Guo, T; Hobbs, DW; Huang, Y; Iserloh, U; Kennedy, ME; Kuvelkar, R; Li, G; Lowrie, J; McHugh, NA; Ozgur, L; Pan, J; Parker, EM; Saionz, K; Stamford, AW; Strickland, C; Tadesse, D; Voigt, J; Wang, L; Wu, Y; Zhang, L; Zhang, Q Rational design of novel, potent piperazinone and imidazolidinone BACE1 inhibitors. Bioorg Med Chem Lett18:3236-41 (2008) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Cathepsin E |
|---|
| Name: | Cathepsin E |
|---|
| Synonyms: | 3.4.23.34 | CATE_HUMAN | CTSE | Cathepsin E form I | Cathepsin E form II |
|---|
| Type: | Enzyme |
|---|
| Mol. Mass.: | 43298.99 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | P14091 |
|---|
| Residue: | 396 |
|---|
| Sequence: | MKTLLLLLLVLLELGEAQGSLHRVPLRRHPSLKKKLRARSQLSEFWKSHNLDMIQFTESC
SMDQSAKEPLINYLDMEYFGTISIGSPPQNFTVIFDTGSSNLWVPSVYCTSPACKTHSRF
QPSQSSTYSQPGQSFSIQYGTGSLSGIIGADQVSVEGLTVVGQQFGESVTEPGQTFVDAE
FDGILGLGYPSLAVGGVTPVFDNMMAQNLVDLPMFSVYMSSNPEGGAGSELIFGGYDHSH
FSGSLNWVPVTKQAYWQIALDNIQVGGTVMFCSEGCQAIVDTGTSLITGPSDKIKQLQNA
IGAAPVDGEYAVECANLNVMPDVTFTINGVPYTLSPTAYTLLDFVDGMQFCSSGFQGLDI
HPPAGPLWILGDVFIRQFYSVFDRGNNRVGLAPAVP
|
|
|
|---|
| BDBM50271626 |
|---|
| n/a |
|---|
| Name | BDBM50271626 |
|---|
| Synonyms: | CHEMBL509210 | N'-[(1S,2S)-2-[(2S)-4-benzyl-3-oxopiperazin-2-yl]-1-(3,5-difluorobenzyl)-2-hydroxyethyl]-5-methyl-N,N-dipropylbenzene-1,3-dicarboxamide | N1-((1S,2S)-1-((S)-4-benzyl-3-oxopiperazin-2-yl)-3-(3,5-difluorophenyl)-1-hydroxypropan-2-yl)-5-methyl-N3,N3-dipropylisophthalamide |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C35H42F2N4O4 |
|---|
| Mol. Mass. | 620.7292 |
|---|
| SMILES | CCCN(CCC)C(=O)c1cc(C)cc(c1)C(=O)N[C@@H](Cc1cc(F)cc(F)c1)[C@H](O)[C@@H]1NCCN(Cc2ccccc2)C1=O |r| |
|---|
| Structure | 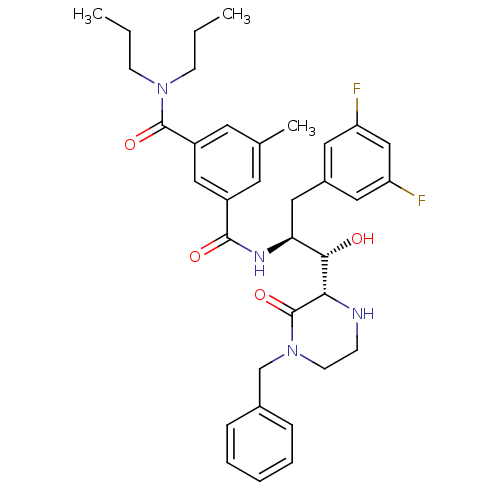
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Cumming, JN; Le, TX; Babu, S; Carroll, C; Chen, X; Favreau, L; Gaspari, P; Guo, T; Hobbs, DW; Huang, Y; Iserloh, U; Kennedy, ME; Kuvelkar, R; Li, G; Lowrie, J; McHugh, NA; Ozgur, L; Pan, J; Parker, EM; Saionz, K; Stamford, AW; Strickland, C; Tadesse, D; Voigt, J; Wang, L; Wu, Y; Zhang, L; Zhang, Q Rational design of novel, potent piperazinone and imidazolidinone BACE1 inhibitors. Bioorg Med Chem Lett18:3236-41 (2008) [PubMed] Article
Cumming, JN; Le, TX; Babu, S; Carroll, C; Chen, X; Favreau, L; Gaspari, P; Guo, T; Hobbs, DW; Huang, Y; Iserloh, U; Kennedy, ME; Kuvelkar, R; Li, G; Lowrie, J; McHugh, NA; Ozgur, L; Pan, J; Parker, EM; Saionz, K; Stamford, AW; Strickland, C; Tadesse, D; Voigt, J; Wang, L; Wu, Y; Zhang, L; Zhang, Q Rational design of novel, potent piperazinone and imidazolidinone BACE1 inhibitors. Bioorg Med Chem Lett18:3236-41 (2008) [PubMed] Article