| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Histamine H4 receptor |
|---|
| Ligand | BDBM50262939 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_489020 (CHEMBL986361) |
|---|
| IC50 | >10000±n/a nM |
|---|
| Citation |  Nagase, T; Mizutani, T; Ishikawa, S; Sekino, E; Sasaki, T; Fujimura, T; Ito, S; Mitobe, Y; Miyamoto, Y; Yoshimoto, R; Tanaka, T; Ishihara, A; Takenaga, N; Tokita, S; Fukami, T; Sato, N Synthesis, structure-activity relationships, and biological profiles of a quinazolinone class of histamine H3 receptor inverse agonists. J Med Chem51:4780-9 (2008) [PubMed] Article Nagase, T; Mizutani, T; Ishikawa, S; Sekino, E; Sasaki, T; Fujimura, T; Ito, S; Mitobe, Y; Miyamoto, Y; Yoshimoto, R; Tanaka, T; Ishihara, A; Takenaga, N; Tokita, S; Fukami, T; Sato, N Synthesis, structure-activity relationships, and biological profiles of a quinazolinone class of histamine H3 receptor inverse agonists. J Med Chem51:4780-9 (2008) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Histamine H4 receptor |
|---|
| Name: | Histamine H4 receptor |
|---|
| Synonyms: | AXOR35 | G-protein coupled receptor 105 | GPCR105 | GPRv53 | HH4R | HISTAMINE H4 | HRH4 | HRH4_HUMAN | Histamine H4 receptor | Histamine H4 receptor (H4R) | Histamine receptor (H3 and H4) | Pfi-013 | SP9144 |
|---|
| Type: | G Protein-Coupled Receptor (GPCR) |
|---|
| Mol. Mass.: | 44517.02 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | Binding assays were using CHO cells stably expressing hH4R receptors. |
|---|
| Residue: | 390 |
|---|
| Sequence: | MPDTNSTINLSLSTRVTLAFFMSLVAFAIMLGNALVILAFVVDKNLRHRSSYFFLNLAIS
DFFVGVISIPLYIPHTLFEWDFGKEICVFWLTTDYLLCTASVYNIVLISYDRYLSVSNAV
SYRTQHTGVLKIVTLMVAVWVLAFLVNGPMILVSESWKDEGSECEPGFFSEWYILAITSF
LEFVIPVILVAYFNMNIYWSLWKRDHLSRCQSHPGLTAVSSNICGHSFRGRLSSRRSLSA
STEVPASFHSERQRRKSSLMFSSRTKMNSNTIASKMGSFSQSDSVALHQREHVELLRARR
LAKSLAILLGVFAVCWAPYSLFTIVLSFYSSATGPKSVWYRIAFWLQWFNSFVNPLLYPL
CHKRFQKAFLKIFCIKKQPLPSQHSRSVSS
|
|
|
|---|
| BDBM50262939 |
|---|
| n/a |
|---|
| Name | BDBM50262939 |
|---|
| Synonyms: | 2-Methyl-3-(4-{[3-(1-pyrrolidinyl)propyl]oxy}phenyl)-5-(trifluoromethyl)-4(3H)-quinazolinone | CHEMBL476323 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C23H24F3N3O2 |
|---|
| Mol. Mass. | 431.4508 |
|---|
| SMILES | Cc1nc2cccc(c2c(=O)n1-c1ccc(OCCCN2CCCC2)cc1)C(F)(F)F |
|---|
| Structure | 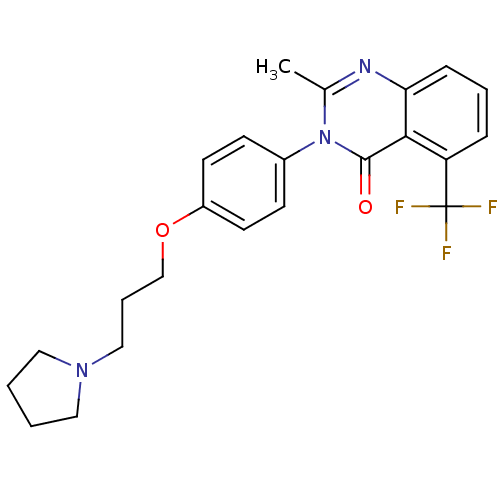
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Nagase, T; Mizutani, T; Ishikawa, S; Sekino, E; Sasaki, T; Fujimura, T; Ito, S; Mitobe, Y; Miyamoto, Y; Yoshimoto, R; Tanaka, T; Ishihara, A; Takenaga, N; Tokita, S; Fukami, T; Sato, N Synthesis, structure-activity relationships, and biological profiles of a quinazolinone class of histamine H3 receptor inverse agonists. J Med Chem51:4780-9 (2008) [PubMed] Article
Nagase, T; Mizutani, T; Ishikawa, S; Sekino, E; Sasaki, T; Fujimura, T; Ito, S; Mitobe, Y; Miyamoto, Y; Yoshimoto, R; Tanaka, T; Ishihara, A; Takenaga, N; Tokita, S; Fukami, T; Sato, N Synthesis, structure-activity relationships, and biological profiles of a quinazolinone class of histamine H3 receptor inverse agonists. J Med Chem51:4780-9 (2008) [PubMed] Article