| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Cholecystokinin receptor type A |
|---|
| Ligand | BDBM50262816 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_490135 (CHEMBL994440) |
|---|
| IC50 | 1.9±n/a nM |
|---|
| Citation |  Zhu, C; Hansen, AR; Bateman, T; Chen, Z; Holt, TG; Hubert, JA; Karanam, BV; Lee, SJ; Pan, J; Qian, S; Reddy, VB; Reitman, ML; Strack, AM; Tong, V; Weingarth, DT; Wolff, MS; MacNeil, DJ; Weber, AE; Duffy, JL; Edmondson, SD Discovery of imidazole carboxamides as potent and selective CCK1R agonists. Bioorg Med Chem Lett18:4393-6 (2008) [PubMed] Article Zhu, C; Hansen, AR; Bateman, T; Chen, Z; Holt, TG; Hubert, JA; Karanam, BV; Lee, SJ; Pan, J; Qian, S; Reddy, VB; Reitman, ML; Strack, AM; Tong, V; Weingarth, DT; Wolff, MS; MacNeil, DJ; Weber, AE; Duffy, JL; Edmondson, SD Discovery of imidazole carboxamides as potent and selective CCK1R agonists. Bioorg Med Chem Lett18:4393-6 (2008) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Cholecystokinin receptor type A |
|---|
| Name: | Cholecystokinin receptor type A |
|---|
| Synonyms: | CCK-A receptor | CCK-AR | CCK1-R | CCKAR | CCKAR_HUMAN | CCKRA | Cholecystokinin receptor | Cholecystokinin receptor type A | Cholecystokinin-1 Receptor |
|---|
| Type: | G Protein-Coupled Receptor (GPCR) |
|---|
| Mol. Mass.: | 47859.34 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | Stable expression of human CCK-1 receptors in HEK 293 cells. |
|---|
| Residue: | 428 |
|---|
| Sequence: | MDVVDSLLVNGSNITPPCELGLENETLFCLDQPRPSKEWQPAVQILLYSLIFLLSVLGNT
LVITVLIRNKRMRTVTNIFLLSLAVSDLMLCLFCMPFNLIPNLLKDFIFGSAVCKTTTYF
MGTSVSVSTFNLVAISLERYGAICKPLQSRVWQTKSHALKVIAATWCLSFTIMTPYPIYS
NLVPFTKNNNQTANMCRFLLPNDVMQQSWHTFLLLILFLIPGIVMMVAYGLISLELYQGI
KFEASQKKSAKERKPSTTSSGKYEDSDGCYLQKTRPPRKLELRQLSTGSSSRANRIRSNS
SAANLMAKKRVIRMLIVIVVLFFLCWMPIFSANAWRAYDTASAERRLSGTPISFILLLSY
TSSCVNPIIYCFMNKRFRLGFMATFPCCPNPGPPGARGEVGEEEEGGTTGASLSRFSYSH
MSASVPPQ
|
|
|
|---|
| BDBM50262816 |
|---|
| n/a |
|---|
| Name | BDBM50262816 |
|---|
| Synonyms: | (4-(isoquinolin-3-yl)piperazin-1-yl)(1-(3-methoxyphenyl)-2-p-tolyl-1H-imidazol-4-yl)methanone | CHEMBL515161 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C31H29N5O2 |
|---|
| Mol. Mass. | 503.5943 |
|---|
| SMILES | COc1cccc(c1)-n1cc(nc1-c1ccc(C)cc1)C(=O)N1CCN(CC1)c1cc2ccccc2cn1 |
|---|
| Structure | 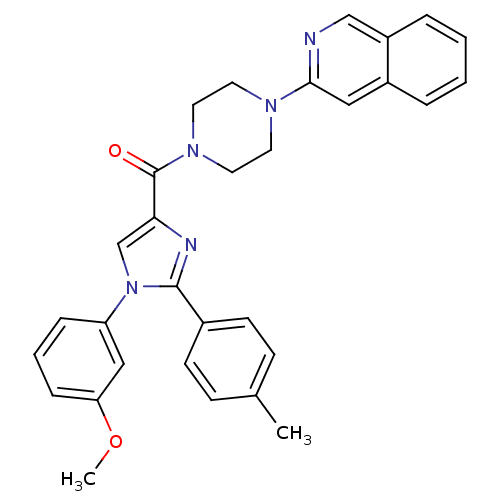
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Zhu, C; Hansen, AR; Bateman, T; Chen, Z; Holt, TG; Hubert, JA; Karanam, BV; Lee, SJ; Pan, J; Qian, S; Reddy, VB; Reitman, ML; Strack, AM; Tong, V; Weingarth, DT; Wolff, MS; MacNeil, DJ; Weber, AE; Duffy, JL; Edmondson, SD Discovery of imidazole carboxamides as potent and selective CCK1R agonists. Bioorg Med Chem Lett18:4393-6 (2008) [PubMed] Article
Zhu, C; Hansen, AR; Bateman, T; Chen, Z; Holt, TG; Hubert, JA; Karanam, BV; Lee, SJ; Pan, J; Qian, S; Reddy, VB; Reitman, ML; Strack, AM; Tong, V; Weingarth, DT; Wolff, MS; MacNeil, DJ; Weber, AE; Duffy, JL; Edmondson, SD Discovery of imidazole carboxamides as potent and selective CCK1R agonists. Bioorg Med Chem Lett18:4393-6 (2008) [PubMed] Article