

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Reaction Details | |||
|---|---|---|---|
 | Report a problem with these data | ||
| Target | Glutamate racemase | ||
| Ligand | BDBM50261912 | ||
| Substrate/Competitor | n/a | ||
| Meas. Tech. | ChEMBL_514356 (CHEMBL967970) | ||
| IC50 | 51±n/a nM | ||
| Citation |  Basarab, GS; Hill, PJ; Rastagar, A; Webborn, PJ Design of Helicobacter pylori glutamate racemase inhibitors as selective antibacterial agents: a novel pro-drug approach to increase exposure. Bioorg Med Chem Lett18:4716-22 (2008) [PubMed] Article Basarab, GS; Hill, PJ; Rastagar, A; Webborn, PJ Design of Helicobacter pylori glutamate racemase inhibitors as selective antibacterial agents: a novel pro-drug approach to increase exposure. Bioorg Med Chem Lett18:4716-22 (2008) [PubMed] Article | ||
| More Info.: | Get all data from this article, Assay Method | ||
| Glutamate racemase | |||
| Name: | Glutamate racemase | ||
| Synonyms: | MURI_HELPY | glr | murI | ||
| Type: | PROTEIN | ||
| Mol. Mass.: | 28413.39 | ||
| Organism: | Helicobacter pylori | ||
| Description: | ChEMBL_475324 | ||
| Residue: | 255 | ||
| Sequence: |
| ||
| BDBM50261912 | |||
| n/a | |||
| Name | BDBM50261912 | ||
| Synonyms: | 2-((6-chloroquinolin-4-yl)methyl)-7-(cyclopropylmethyl)-5-methyl-3-(2-methyl-5-(methylsulfonyl)furan-3-yl)-2H-pyrazolo[3,4-d]pyrimidine-4,6(5H,7H)-dione | CHEMBL469008 | ||
| Type | Small organic molecule | ||
| Emp. Form. | C26H24ClN5O5S | ||
| Mol. Mass. | 554.017 | ||
| SMILES | Cc1oc(cc1-c1n(Cc2ccnc3ccc(Cl)cc23)nc2n(CC3CC3)c(=O)n(C)c(=O)c12)S(C)(=O)=O |(-3.85,-28.84,;-2.31,-28.84,;-1.41,-27.59,;.05,-28.06,;.05,-29.6,;-1.41,-30.08,;-1.88,-31.55,;-.97,-32.8,;.57,-32.8,;1.34,-34.14,;.57,-35.47,;1.34,-36.8,;2.88,-36.8,;3.65,-35.46,;5.18,-35.45,;5.94,-34.12,;5.16,-32.79,;5.92,-31.45,;3.63,-32.8,;2.88,-34.13,;-1.88,-34.06,;-3.36,-33.58,;-4.7,-34.34,;-4.7,-35.89,;-6.04,-36.65,;-7.58,-36.66,;-6.81,-37.99,;-6.04,-33.57,;-7.37,-34.34,;-6.04,-32.02,;-7.37,-31.26,;-4.7,-31.25,;-4.7,-29.7,;-3.36,-32.02,;1.29,-27.15,;2.62,-26.37,;.45,-25.87,;2.14,-28.44,)| | ||
| Structure | 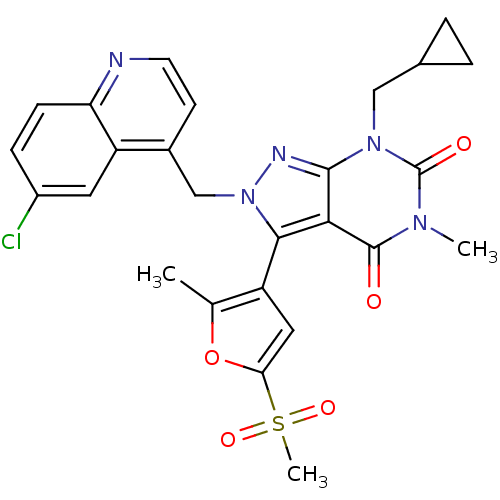
| ||